Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence
- PMID: 9990004
- PMCID: PMC15443
- DOI: 10.1073/pnas.96.4.1218
Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence
Abstract
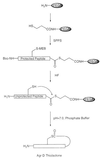
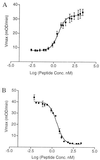
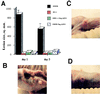
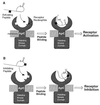
The synthesis of virulence factors and other extracellular proteins responsible for pathogenicity in Staphylococcus aureus is under the control of the agr locus. A secreted agr-encoded peptide, AgrD, processed from the AgrD gene product, is known to be an effector of self-strain activation and cross-strain inhibition of the agr response. Biochemical analysis of AgrD peptides isolated from culture supernatants has suggested that they contain an unusual thiol ester-linked cyclic structure. In the present work, chemical synthesis is used to confirm that the mature AgrD peptides contain a thiolactone structure and that this feature is absolutely necessary for full biological activity. The AgrD synthetic thiolactone peptides exhibited biological activity in vivo in a mouse protection test. Structure-activity studies have allowed key aspects of the peptide structure involved in the differential activation and inhibition functions to be identified. Accordingly, we propose a model for activation and inhibition of the agr response in which the former, but not the latter, involves specific acylation of the agr transmembrane receptor, AgrC.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

