Mechanism of biological synergy between cellular Src and epidermal growth factor receptor
- PMID: 9990038
- PMCID: PMC15477
- DOI: 10.1073/pnas.96.4.1415
Mechanism of biological synergy between cellular Src and epidermal growth factor receptor
Abstract
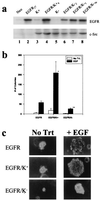
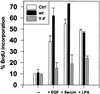
Overexpression of both cellular Src (c-Src) and the epidermal growth factor receptor (EGFR) occurs in many of the same human tumors, suggesting that they may functionally interact and contribute to the progression of cancer. Indeed, in murine fibroblasts, overexpression of c-Src has been shown to potentiate the mitogenic and tumorigenic capacity of the overexpressed EGFR. Potentiation correlated with the ability of c-Src to physically associate with the activated EGFR and the appearance of two unique in vivo phosphorylations on the receptor (Tyr-845 and Tyr-1101). Using stable cell lines of C3H10T1/2 murine fibroblasts that contain kinase-deficient (K-) c-Src and overexpressed wild-type EGFR, we show that the kinase activity of c-Src is required for both the biological synergy with the receptor and the phosphorylations on the receptor, but not for the association of c-Src with the receptor. In transient transfection assays, not only epidermal growth factor but also serum- and lysophosphatidic acid-induced DNA synthesis was ablated in a dominant-negative fashion by a Y845F mutant of the EGFR, indicating that c-Src-induced phosphorylation of Y845 is critical for the mitogenic response to both the EGFR and a G protein-coupled receptor (lysophosphatidic acid receptor). Unexpectedly, the Y845F mutant EGFR was found to retain its full kinase activity and its ability to activate the adapter protein SHC and extracellular signal-regulated kinase ERK2 in response to EGF, demonstrating that the mitogenic pathway involving phosphorylation of Y845 is independent of ERK2-activation. The application of these findings to the development of novel therapeutics for human cancers that overexpress c-Src and EGFR is discussed.
Figures




References
-
- Ottenhoff-Kalff A E, Rijksen G, van Beurden E A, Hennipman A, Michels A A, Staal G E. Cancer Res. 1992;52:4773–4778. - PubMed
-
- Khazaie K, Schirrmacher V, Lichtner R B. Cancer Metastasis Rev. 1993;12:255–274. - PubMed
-
- Banker N, Evers B M, Hellmich M R, Townsend C J. Surg Oncol. 1996;5:201–210. - PubMed
-
- Biscardi, J. S., Tice, D. A. & Parsons, S. J. (1999) Adv. Cancer Res. 76, in press. - PubMed
-
- Harris J R, Lippmann M E, Veronesi U, Willett W. N Engl J Med. 1992;327:473–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

