Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes
- PMID: 9990041
- PMCID: PMC15480
- DOI: 10.1073/pnas.96.4.1433
Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes
Abstract
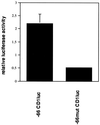
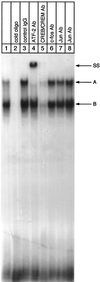
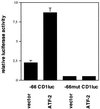
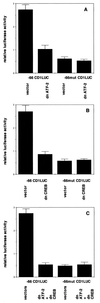
Endochondral bone growth is regulated by the rates of chondrocyte proliferation and differentiation. However, the intracellular mechanisms regulating these processes are poorly understood. Recently, interruption of the gene encoding the transcription factor activating transcription factor 2 (ATF-2) was shown to inhibit proliferation of chondrocytes in mice [Reimold, A. M., et al. (1996) Nature (London) 379, 262-265]. The target genes of ATF-2 that are responsible for this phenotype remain unknown. Here we report that the cyclin D1 gene is a direct target of ATF-2 in chondrocytes. ATF-2 is present in nuclear extracts from chondrogenic cell lines and binds, as a complex with a CRE-binding protein (CREB)/CRE modulator protein, to the cAMP response element (CRE) in the cyclin D1 promoter. Mutation of the cyclin D1 CRE caused a 78% reduction in the activity of the promoter in chondrocytes. Overexpression of ATF-2 in chondrocytes enhanced activity of the cyclin D1 promoter 3. 5-fold. In contrast, inhibition of endogenous ATF-2 or CREB by expression of dominant-negative inhibitors of CREB and ATF-2 significantly reduced the activity of the promoter in chondrocytes through the CRE. In addition, levels of cyclin D1 protein are greatly reduced in the chondrocytes of ATF-2-deficient mice. These data identify the cyclin D1 gene as a direct target of ATF-2 in chondrocytes and suggest that reduced expression of cyclin D1 contributes to the defective cartilage development of these mice.
Figures


References
-
- Mundlos S, Olsen B R. FASEB J. 1997;11:227–233. - PubMed
-
- Mundlos S, Olsen B R. FASEB J. 1997;11:125–132. - PubMed
-
- Reimold A M, Grusby M J, Kosaras B, Fries J W V, Mori R, Maniwa S, Clauss I M, Collins T, Sidman R L, Glimcher M J, et al. Nature (London) 1996;379:262–265. - PubMed
-
- Landshultz W H, Johnson P F, McKnight S L. Science. 1988;240:1759–1764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

