In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria
- PMID: 9990078
- PMCID: PMC15546
- DOI: 10.1073/pnas.96.4.1645
In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria
Abstract
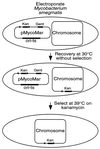
mariner family transposons are widespread among eukaryotic organisms. These transposons are apparently horizontally transmitted among diverse eukaryotes and can also transpose in vitro in the absence of added cofactors. Here we show that transposons derived from the mariner element Himar1 can efficiently transpose in bacteria in vivo. We have developed simple transposition systems by using minitransposons, made up of short inverted repeats flanking antibiotic resistance markers. These elements can efficiently transpose after expression of transposase from an appropriate bacterial promoter. We found that transposition of mariner-based elements in Escherichia coli produces diverse insertion mutations in either a targeted plasmid or a chromosomal gene. With Himar1-derived transposons we were able to isolate phage-resistant mutants of both E. coli and Mycobacterium smegmatis. mariner-based transposons will provide valuable tools for mutagenesis and genetic manipulation of bacteria that currently lack well developed genetic systems.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

