The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro
- PMID: 9990852
- PMCID: PMC316433
- DOI: 10.1101/gad.13.3.270
The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro
Erratum in
- Genes Dev 1999 Apr 15;13(8):1050
Abstract
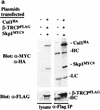
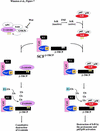
Ubiquitin-mediated proteolysis has a central role in controlling the intracellular levels of several important regulatory molecules such as cyclins, CKIs, p53, and IkappaBalpha. Many diverse proinflammatory signals lead to the specific phosphorylation and subsequent ubiquitin-mediated destruction of the NF-kappaB inhibitor protein IkappaBalpha. Substrate specificity in ubiquitination reactions is, in large part, mediated by the specific association of the E3-ubiquitin ligases with their substrates. One class of E3 ligases is defined by the recently described SCF complexes, the archetype of which was first described in budding yeast and contains Skp1, Cdc53, and the F-box protein Cdc4. These complexes recognize their substrates through modular F-box proteins in a phosphorylation-dependent manner. Here we describe a biochemical dissection of a novel mammalian SCF complex, SCFbeta-TRCP, that specifically recognizes a 19-amino-acid destruction motif in IkappaBalpha (residues 21-41) in a phosphorylation-dependent manner. This SCF complex also recognizes a conserved destruction motif in beta-catenin, a protein with levels also regulated by phosphorylation-dependent ubiquitination. Endogenous IkappaBalpha-ubiquitin ligase activity cofractionates with SCFbeta-TRCP. Furthermore, recombinant SCFbeta-TRCP assembled in mammalian cells contains phospho-IkappaBalpha-specific ubiquitin ligase activity. Our results suggest that an SCFbeta-TRCP complex functions in multiple transcriptional programs by activating the NF-kappaB pathway and inhibiting the beta-catenin pathway.
Figures










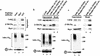



References
-
- Bai C, Sen P, Mathias N, Hofmann K, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulation to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. - PubMed
-
- Baeuerle PA, Baltimore D. NF-κB: Ten years after. Cell. 1996;87:13–20. - PubMed
-
- Beg AA, Baltimore D. An essential role for NF-κB in preventing TNFα-induced cell death. Science. 1996;274:782–784. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
