PCR-reverse dot blot human papillomavirus genotyping as a primary screening test for cervical cancer in a hospital-based cohort
- PMID: 30887754
- PMCID: PMC6424850
- DOI: 10.3802/jgo.2019.30.e29
PCR-reverse dot blot human papillomavirus genotyping as a primary screening test for cervical cancer in a hospital-based cohort
Abstract
Objective: To evaluate the polymerase chain reaction (PCR)-reverse-dot-blot (RDB) human papillomavirus (HPV) genotyping test as a feasible assay for the cervical cancer primary screening.
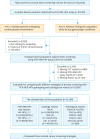
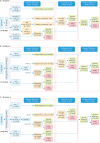
Methods: In a hospital-based cohort, a total of 21,568 women were voluntarily enrolled from March 2009 to November 2016 for evaluating the 3 current cervical cancer screening strategies: co-test, cytology primary and high-risk HPV (HR-HPV) primary by using PCR-RDB HPV genotyping and liquid-based cytology (thinprep cytologic test [TCT]). Women with HR-HPV infection and/or abnormal cytology were referred for colposcopy, and the biopsy or conization was performed according to the American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines.
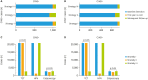
Results: Overall, 18.20% (3,935/21,568) of the women were detected as HR-HPV-positive, 5.04% (1,088/21,568) were diagnosed with cervical intraepithelial neoplasia 2 or higher (CIN2+), and 3.43% (739/21,568) with CIN3+. The cumulative incidence rates for CIN2+/CIN3+ in patients with HPV-16/18-positive were 48.28%/37.20%, while they were 0.86%/0.38%, 0.30%/0.15% and 0.18%/0.09% in cytology-negative, HR-HPV-negative and co-test-negative population, respectively. Using CIN2+ and CIN3+ as the observed endpoints, the sensitivity and negative predictive value (NPV) of HR-HPV genotyping as a primary screening tool were 90.99%/99.49% and 91.57%/99.80%. Moreover, using HR-HPV genotyping primary screening could detect the same more CIN2+/CIN3+ cases in baseline-detection as co-testing (990/700 vs. 991/701) and far more than cytology primary screening (903/656, p<0.05). It also achieved the lowest misdiagnosis rate (8.01%/5.02%). Although HPV genotyping primary screening required an increased number of colposcopies (2.75/3.89 per CIN2+/CIN3+ case), it yielded an acceptable rate.
Conclusions: The PCR-RDB HPV genotyping test is a cost-effective and beneficial cervical cancer primary screening for hospital-based opportunistic screening.
Keywords: Cancer Screening; Cervical Cancer; Cytology; Genotype; Papillomaviridae.
Copyright © 2019. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- International Agency for Research on Cancer. GLOBOCAN 2012: cancer fact sheet. Cervical cancer estimated incidence, mortality and prevalence worldwide in 2012 [Internet] Lyon: International Agency for Research on Cancer; 2013. [cited 2013 Dec 12]. Available from: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp.
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. - PubMed
-
- Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382:889–899. - PubMed

