Inhibition of PDE4 Attenuates TNF-α-Triggered Cell Death Through Suppressing NF-κB and JNK Activation in HT-22 Neuronal Cells
- PMID: 31659561
- PMCID: PMC11448866
- DOI: 10.1007/s10571-019-00745-w
Inhibition of PDE4 Attenuates TNF-α-Triggered Cell Death Through Suppressing NF-κB and JNK Activation in HT-22 Neuronal Cells
Abstract
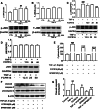
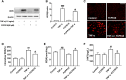
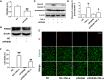
Tumor necrosis factor-α (TNF-α) is a critical pro-inflammatory cytokine regulating neuroinflammation. At high concentrations, it is toxic to neurons, and such damage is positively correlated with acute and chronic neurological diseases. Our previous studies showed that inhibition of phosphodiesterase 4 (PDE4) attenuated the production of TNF-α induced by lipopolysaccharides in microglial cells. However, whether PDE4 inhibition can block the neurotoxic effects of TNF-α in neuronal cells is unknown. In this study, we investigated the protective effects of FCPR16, a novel PDE4 inhibitor, against TNF-α-induced cellular apoptosis in HT-22 hippocampal neuronal cells. We demonstrated that FCPR16 dose-dependently increased the viability of HT-22 cells exposed to TNF-α insult. Propidium iodide/calcein staining and flow cytometry analysis showed that FCPR16 decreased cell apoptosis triggered by TNF-α. Western blot analysis showed that FCPR16 decreased the level of cleaved caspase 3 and caspase 8, but had no effect on caspase 9. Mechanistically, FCPR16 blocked the TNF-α-induced phosphorylation of c-Jun N-terminal kinase (JNK) in HT-22 cells, and inhibition of JNK showed a similar protective effect as FCPR16. Furthermore, FCPR16 decreased the translocation of nuclear factor-κB (NF-κB) p65 from the cytosol into the nucleus. In addition, FCPR16 decreased the expression of inducible nitric oxide synthase and the production of reactive oxygen species in HT-22 cells exposed to TNF-α. Moreover, knockdown of PDE4B by specific small interfering RNA reduced the apoptosis of HT-22 cells treated with TNF-α. Taken together, our findings suggest that FCPR16 promotes the survival of neuronal cells exposed to TNF-α by suppressing the activation of JNK and NF-κB.
Keywords: FCPR16; JNK; NF-κB; Neuroinflammation; Phosphodiesterase 4.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Aggarwal BB (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3(9):745–756 - PubMed
-
- Annibaldi A, Meier P (2018) Checkpoints in TNF-induced cell death: implications in inflammation and cancer. Trends Mol Med 24(1):49–65 - PubMed
-
- Bolger GB (2017) The PDE4 cAMP-specific phosphodiesterases: targets for drugs with antidepressant and memory-enhancing action. Adv Neurobiol 17:63–102 - PubMed
-
- Borghi A, Verstrepen L, Beyaert R (2016) TRAF2 multitasking in TNF receptor-induced signaling to NF-kappaB, MAP kinases and cell death. Biochem Pharmacol 116:1–10 - PubMed
MeSH terms
Substances
Grants and funding
- 81773698/National Natural Science Foundation of China
- IRT_16R37/Program for Changjiang Scholars and Innovative Research Team in University
- 2018B030334001/Science and Technology Program of Guangdong
- 2015B020211007/Science and Technology Program of Guangdong
- 201604020112/Science and Technology Program of Guangzhou
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

