Loss of ZBED6 Protects Against Sepsis-Induced Muscle Atrophy by Upregulating DOCK3-Mediated RAC1/PI3K/AKT Signaling Pathway in Pigs
- PMID: 37551034
- PMCID: PMC10582467
- DOI: 10.1002/advs.202302298
Loss of ZBED6 Protects Against Sepsis-Induced Muscle Atrophy by Upregulating DOCK3-Mediated RAC1/PI3K/AKT Signaling Pathway in Pigs
Abstract
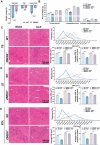
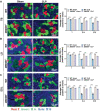
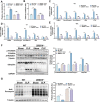
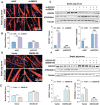
Sepsis-induced muscle atrophy often increases morbidity and mortality in intensive care unit (ICU) patients, yet neither therapeutic target nor optimal animal model is available for this disease. Here, by modifying the surgical strategy of cecal ligation and puncture (CLP), a novel sepsis pig model is created that for the first time recapitulates the whole course of sepsis in humans. With this model and sepsis patients, increased levels of the transcription factor zinc finger BED-type containing 6 (ZBED6) in skeletal muscle are shown. Protection against sepsis-induced muscle wasting in ZBED6-deficient pigs is further demonstrated. Mechanistically, integrated analysis of RNA-seq and ChIP-seq reveals dedicator of cytokinesis 3 (DOCK3) as the direct target of ZBED6. In septic ZBED6-deficient pigs, DOCK3 expression is increased in skeletal muscle and myocytes, activating the RAC1/PI3K/AKT pathway and protecting against sepsis-induced muscle wasting. Conversely, opposite gene expression patterns and exacerbated muscle wasting are observed in septic ZBED6-overexpressing myotubes. Notably, sepsis patients show increased ZBED6 expression along with reduced DOCK3 and downregulated RAC1/PI3K/AKT pathway. These findings suggest that ZBED6 is a potential therapeutic target for sepsis-induced muscle atrophy, and the established sepsis pig model is a valuable tool for understanding sepsis pathogenesis and developing its therapeutics.
Keywords: DOCK3; ZBED6; muscle atrophy; sepsis.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bercker S., Weber‐Carstens S., Deja M., Grimm C., Wolf S., Behse F., Busch T., Falke K. J., Kaisers U., Crit. Care Med. 2005, 33, 711. - PubMed
-
- a) Hahn A., Kny M., Pablo‐Tortola C., Todiras M., Willenbrock M., Schmidt S., Schmoeckel K., Jorde I., Nowak M., Jarosch E., Sommer T., Broker B. M., Felix S. B., Scheidereit C., Weber‐Carstens S., Butter C., Luft F. C., Fielitz J., J Cachexia Sarcopenia Muscle 2020, 11, 103; - PMC - PubMed
- b) Zanders L., Kny M., Hahn A., Schmidt S., Wundersitz S., Todiras M., Lahmann I., Bandyopadhyay A., Wollersheim T., Kaderali L., Luft F. C., Birchmeier C., Weber‐Carstens S., Fielitz J., J Cachexia Sarcopenia Muscle 2022, 13, 713; - PMC - PubMed
- c) Lang C. H., Shock 2022, 59, 214. - PubMed
-
- a) Rocheteau P., Chatre L., Briand D., Mebarki M., Jouvion G., Bardon J., Crochemore C., Serrani P., Lecci P. P., Latil M., Matot B., Carlier P. G., Latronico N., Huchet C., Lafoux A., Sharshar T., Ricchetti M., Chretien F., Nat. Commun. 2015, 6, 10145; - PMC - PubMed
- b) Kemp P. R., Paul R., Hinken A. C., Neil D., Russell A., Griffiths M. J., J Cachexia Sarcopenia Muscle 2020, 11, 1321. - PMC - PubMed
-
- a) Hoover D. B., Ozment T. R., Wondergem R., Li C., Williams D. L., Shock 2015, 43, 185; - PMC - PubMed
- b) Haines R. W., Zolfaghari P., Wan Y., Pearse R. M., Puthucheary Z., Prowle J. R., Intensive Care Med 2019, 45, 1718; - PubMed
- c) Chapple L. S., Kouw I. W. K., Summers M. J., Weinel L. M., Gluck S., Raith E., Slobodian P., Soenen S., Deane A. M., van Loon L. J. C., Chapman M. J., Am J. Respir. Crit. Care Med. 2022, 206, 740; - PubMed
- d) Puthucheary Z., Rooyackers O., Am J. Respir. Crit. Care Med. 2022, 206, 660; - PMC - PubMed
- e) Steiner J. L., Lang C. H., Shock 2015, 43, 344; - PMC - PubMed
- f) van Hees H. W., Schellekens W. J., Linkels M., Leenders F., Zoll J., Donders R., Dekhuijzen P. N., van der Hoeven J. G., Heunks L. M., Crit. Care 2011, 15, R233. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2021YFF1000602/National Key Research and Development Program of China
- 2023-CX-TD-57/Program for Shaanxi Science and Technology from Shaanxi Provincial Science and Technology Department
- 2021ZDZX0008/Sichuan Science and Technology Program
- 2021YFYZ0009/Sichuan Science and Technology Program
- 32225046/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
