Mesenchymal stem cells from perinatal tissues promote diabetic wound healing via PI3K/AKT activation
- PMID: 39923118
- PMCID: PMC11807333
- DOI: 10.1186/s13287-025-04141-8
Mesenchymal stem cells from perinatal tissues promote diabetic wound healing via PI3K/AKT activation
Abstract
Background: Diabetic foot ulcers (DFUs) represent a major complication of diabetes, often leading to poor healing outcomes with conventional treatments. Mesenchymal stem cell (MSC) therapies have emerged as a promising alternative, given their potential to modulate various pathways involved in wound healing. This study evaluates and compares the therapeutic potential of MSCs derived from perinatal tissues-human umbilical cord MSCs (hUCMSCs), human chorionic villi MSCs (hCVMSCs), and human decidua basalis MSCs (hDCMSCs)-in a diabetic wound healing model.
Methods: We performed in vitro and in vivo studies to compare the efficacy of hUCMSCs, hCVMSCs, and hDCMSCs. Mass spectrometry was used to analyze the secreted proteins of the MSCs. We incorporated the MSCs into a polyethylene glycol diacrylate (PEGDA) and sodium alginate (SA) hydrogel matrix with collagen I (Col-I) to evaluate their effects on wound healing.
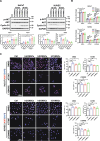
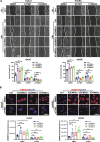
Results: All three types of MSCs promoted wound healing, with hUCMSCs and hCVMSCs showing stronger effects compared to hDCMSCs. Both hUCMSCs and hCVMSCs demonstrated robust wound healing kinetics, with enhanced keratinocyte proliferation (KRT14+/Ki67+ cells), maturation (KRT10/KRT14 ratio), and angiogenesis. In vitro studies demonstrated that the MSC-derived secretome enhanced keratinocyte proliferation and migration, endothelial cell function and stem cell recruitment, indicating robust paracrine effects. Mass spectrometry revealed a conserved set of proteins including THBS1 (thrombospondin 1), SERPINE1 (serpin family E member 1), ANXA1 (annexin A1), LOX (lysyl oxidase), and ITGB1 (integrin beta-1) which are involved in extracellular matrix (ECM) organization and wound healing, with the PI3K/AKT signaling pathway playing a central role. The PEGDA/SA/Col-I hydrogel demonstrated a unique balance of mechanical and biological properties and an optimal environment for MSC viability and function. Application of either hUCMSC- or hCVMSC-laden hydrogels resulted in accelerated wound closure, improved re-epithelialization, increased collagen deposition, and enhanced vascularization in vivo.
Conclusions: MSCs From perinatal tissues particularly hUCMSCs and hCVMSCs significantly enhance diabetic wound healing through PI3K/AKT pathway activation while hDCMSCs exhibited weaker efficacy. The PEGDA/SA/Col-I hydrogel supports MSC viability and function offering a promising scaffold for DFU treatment. These findings underscore the potential of specific perinatal MSCs and optimized hydrogel formulations in advancing diabetic wound care.
Keywords: Diabetic wound healing; Hydrogel; MSCs; PI3K/AKT; Placenta.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Isolation of human umbilical cord- and placenta-derived MSCs was approved by the Joint CUHK-NTEC Clinical Research Ethics Committee (Title: Placenta derived genes analysis in placenta and maternal circulation in singleton pregnancies complicated with fetal growth restriction; Ref: 2020.313; Year of approval: 2020). The use of human bone marrow for MSC isolation was approved by Joint CUHK-NTEC Clinical Research Ethics Committee (Title: A novel preclinical treatment strategy for enhancing chemosensitivity of pediatric B-precursor acute lymphoblastic leukemia by disruption of leukemia-stroma interaction using CD9-neutralizing antibody in a mouse model; Ref: 2015.018; Year of approval: 2015). Written informed consent for participation in the study or the use of samples was provided to the patients or their guardians. All animal use and research protocols in this study were approved by the Laboratory Animal Experimentation Ethical Committee, Chinese University of Hong Kong (Title: Development of stem cell-based therapy for diabetic foot ulcer (DFU); Ref: 21–162-MIS; Year of approval: 2021) and were in accordance with the Guideline for the Care and Use of Laboratory Animals. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

