Design, Synthesis, and Antiproliferative Activity of Novel Neocryptolepine-Rhodanine Hybrids
- PMID: 36364427
- PMCID: PMC9656124
- DOI: 10.3390/molecules27217599
Design, Synthesis, and Antiproliferative Activity of Novel Neocryptolepine-Rhodanine Hybrids
Abstract
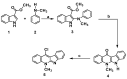
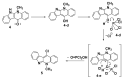
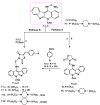
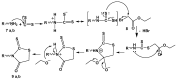
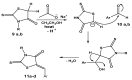
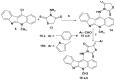
A series of novel neocryptolepine-rhodanine hybrids (9a,b, 11a-d, 14, and 16a,b) have been synthesized by combining neocryptolepine core 5 modified at the C-11 position with rhodanine condensed with the appropriate aryl/hetero aryl aldehydes. Based on these findings, the structures of the hybrids were confirmed by spectral analyses. By employing the MTT assay, all hybrids were tested for their in vitro antiproliferative activity against two cancer cell lines, including MDA-MB-231 (human breast) and HepG-2 (hepatocellular carcinoma). Interestingly, the IC50 values of all hybrids except 9b and 11c showed activity comparable to the standard anticancer drug, 5-fluorouracil, against HepG-2 cancer cells. Furthermore, the cytotoxicity of all the synthesized hybrids was investigated on a normal skin human cell line (BJ-1), and the results showed that these compounds had no significant cytotoxicity toward these healthy cells at the highest concentration used in this study. This study also indicated that the active hybrids exert their cytotoxic activity via the induction of apoptosis. A molecular docking study was used to shed light on the molecular mechanism of their anticancer activity. The docking results revealed that the hybrids exert their mode of action through DNA intercalation. Furthermore, in silico assessment for pharmacokinetic properties was performed on the most potent compounds, which revealed candidates with good bioavailability, high tolerability with cell membranes, and positive drug-likeness values.
Keywords: alkaloids; docking; hybrids; neocryptolepine; pharmacokinetics; rhodanine.
Conflict of interest statement
There is no conflict to declare.
Figures









References
-
- Sharaf M.H.H., Schiff P.L., Tackie J.A.N., Phoebe C.H., Johnson J.R.L., Minick D., Andrews C.W., Crouch R.C., Martin G.E. The isolation and structure determination of cryptomisrine, a novel indolo[3,2-b]quinoline dimeric alkaloid from CryptolePis sanguinolenta. J. Heterocycl. Chem. 1996;33:789–797. doi: 10.1002/jhet.5570330343. - DOI
-
- Boye G.L., Ampofo O. Clinical uses of CryptolePis sanguinolenta (AsclePiadaceae); Proceedings of the 1st International Seminar on CryptolePine, University of Science and Technology; Kumasi, Ghana. 1983. pp. 37–40.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

