A Rapid, Simple, Inexpensive, and Mobile Colorimetric Assay COVID-19-LAMP for Mass On-Site Screening of COVID-19
- PMID: 32751106
- PMCID: PMC7432162
- DOI: 10.3390/ijms21155380
A Rapid, Simple, Inexpensive, and Mobile Colorimetric Assay COVID-19-LAMP for Mass On-Site Screening of COVID-19
Abstract
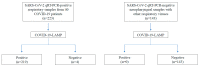
To control the COVID-19 pandemic and prevent its resurgence in areas preparing for a return of economic activities, a method for a rapid, simple, and inexpensive point-of-care diagnosis and mass screening is urgently needed. We developed and evaluated a one-step colorimetric reverse-transcriptional loop-mediated isothermal amplification assay (COVID-19-LAMP) for detection of SARS-CoV-2, using SARS-CoV-2 isolate and respiratory samples from patients with COVID-19 (n = 223) and other respiratory virus infections (n = 143). The assay involves simple equipment and techniques and low cost, without the need for expensive qPCR machines, and the result, indicated by color change, is easily interpreted by naked eyes. COVID-19-LAMP can detect SARS-CoV-2 RNA with detection limit of 42 copies/reaction. Of 223 respiratory samples positive for SARS-CoV-2 by qRT-PCR, 212 and 219 were positive by COVID-19-LAMP at 60 and 90 min (sensitivities of 95.07% and 98.21%) respectively, with the highest sensitivities among nasopharyngeal swabs (96.88% and 98.96%), compared to sputum/deep throat saliva samples (94.03% and 97.02%), and throat swab samples (93.33% and 98.33%). None of the 143 samples with other respiratory viruses were positive by COVID-19-LAMP, showing 100% specificity. Samples with higher viral load showed shorter detection time, some as early as 30 min. This inexpensive, highly sensitive and specific COVID-19-LAMP assay can be useful for rapid deployment as mobile diagnostic units to resource-limiting areas for point-of-care diagnosis, and for unlimited high-throughput mass screening at borders to reduce cross-regional transmission.
Keywords: COVID-19; RT-LAMP; SARS-CoV-2; diagnosis; mass screening; mobile Diagnostic; on-site screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19.Med Hypotheses. 2020 Aug;141:109786. doi: 10.1016/j.mehy.2020.109786. Epub 2020 Apr 25. Med Hypotheses. 2020. PMID: 32361529 Free PMC article.
-
iSCAN: An RT-LAMP-coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-2.Virus Res. 2020 Oct 15;288:198129. doi: 10.1016/j.virusres.2020.198129. Epub 2020 Aug 18. Virus Res. 2020. PMID: 32822689 Free PMC article.
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
SARS-CoV-2 detection in different respiratory sites: A systematic review and meta-analysis.EBioMedicine. 2020 Sep;59:102903. doi: 10.1016/j.ebiom.2020.102903. Epub 2020 Jul 24. EBioMedicine. 2020. PMID: 32718896 Free PMC article.
-
Point-of-Care Diagnostics of COVID-19: From Current Work to Future Perspectives.Sensors (Basel). 2020 Jul 31;20(15):4289. doi: 10.3390/s20154289. Sensors (Basel). 2020. PMID: 32752043 Free PMC article. Review.
Cited by
-
Diagnostic accuracy of LAMP versus PCR over the course of SARS-CoV-2 infection.Int J Infect Dis. 2021 Jun;107:195-200. doi: 10.1016/j.ijid.2021.04.018. Epub 2021 Apr 20. Int J Infect Dis. 2021. PMID: 33862213 Free PMC article.
-
Development of loop-mediated isothermal amplification (LAMP) assays using five primers reduces the false-positive rate in COVID-19 diagnosis.Sci Rep. 2023 Mar 28;13(1):5066. doi: 10.1038/s41598-023-31760-z. Sci Rep. 2023. PMID: 36977756 Free PMC article.
-
Essential properties and pitfalls of colorimetric Reverse Transcription Loop-mediated Isothermal Amplification as a point-of-care test for SARS-CoV-2 diagnosis.Mol Med. 2021 Mar 26;27(1):30. doi: 10.1186/s10020-021-00289-0. Mol Med. 2021. PMID: 33771097 Free PMC article.
-
Visual Detection of COVID-19 from Materials Aspect.Adv Fiber Mater. 2022;4(6):1304-1333. doi: 10.1007/s42765-022-00179-y. Epub 2022 Aug 8. Adv Fiber Mater. 2022. PMID: 35966612 Free PMC article. Review.
-
Development and Validation of a Highly Sensitive RT-qLAMP Assay for Rapid Detection of SARS-CoV-2: Methodological Aspects.Mol Biotechnol. 2024 Sep 24. doi: 10.1007/s12033-024-01275-7. Online ahead of print. Mol Biotechnol. 2024. PMID: 39316362
References
-
- Lau S.K., Feng Y., Chen H., Luk H.K., Yang W.H., Li K.S., Zhang Y.Z., Huang Y., Song Z.Z., Chow W.N., et al. Severe Acute Respiratory Syndrome (SARS) Coronavirus ORF8 Protein Is Acquired from SARS-Related Coronavirus from Greater Horseshoe Bats through Recombination. J. Virol. 2015;89:10532–10547. doi: 10.1128/JVI.01048-15. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

