Sensitive fluorescence detection of SARS-CoV-2 RNA in clinical samples via one-pot isothermal ligation and transcription
- PMID: 32948855
- PMCID: PMC7499000
- DOI: 10.1038/s41551-020-00617-5
Sensitive fluorescence detection of SARS-CoV-2 RNA in clinical samples via one-pot isothermal ligation and transcription
Abstract
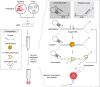
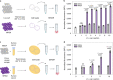
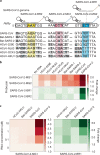
The control of viral outbreaks requires nucleic acid diagnostic tests that are sensitive, simple and fast. Here, we report a highly sensitive and specific one-pot assay for the fluorescence-based detection of RNA from pathogens. The assay, which can be performed within 30-50 min of incubation time and can reach a limit of detection of 0.1-attomolar RNA concentration, relies on a sustained isothermal reaction cascade producing an RNA aptamer that binds to a fluorogenic dye. The RNA aptamer is transcribed by the T7 RNA polymerase from the ligation product of a promoter DNA probe and a reporter DNA probe that hybridize with the target single-stranded RNA sequence via the SplintR ligase (a Chlorella virus DNA ligase). In 40 nasopharyngeal SARS-CoV-2 samples, the assay reached positive and negative predictive values of 95 and 100%, respectively. We also show that the assay can rapidly detect a range of viral and bacterial RNAs.
Conflict of interest statement
Patent applications have been submitted by J.W.L., G.Y.J., C.H.W., S.J. and G.S. based on the results of this study (PCT/KR2020/005331 (Patent Cooperation Treaty) and 10-2020-0048912 (Republic of Korea)).
Figures





Comment in
-
Rapid and frequent testing.Nat Biomed Eng. 2020 Dec;4(12):1121-1122. doi: 10.1038/s41551-020-00670-0. Nat Biomed Eng. 2020. PMID: 33293723 No abstract available.
Similar articles
-
Split T7 promoter-based isothermal transcription amplification for one-step fluorescence detection of SARS-CoV-2 and emerging variants.Biosens Bioelectron. 2022 Jul 15;208:114221. doi: 10.1016/j.bios.2022.114221. Epub 2022 Mar 31. Biosens Bioelectron. 2022. PMID: 35421842 Free PMC article.
-
Clinical validation of a Cas13-based assay for the detection of SARS-CoV-2 RNA.Nat Biomed Eng. 2020 Dec;4(12):1140-1149. doi: 10.1038/s41551-020-00603-x. Epub 2020 Aug 26. Nat Biomed Eng. 2020. PMID: 32848209
-
Colorimetric reverse transcription loop-mediated isothermal amplification (RT-LAMP) as a visual diagnostic platform for the detection of the emerging coronavirus SARS-CoV-2.Analyst. 2021 Jan 21;146(2):471-477. doi: 10.1039/d0an01775b. Epub 2020 Nov 9. Analyst. 2021. PMID: 33165486
-
Real-time detection of SARS-CoV-2 in clinical samples via ultrafast ligation-dependent RNA transcription amplification.Anal Methods. 2023 Apr 13;15(15):1915-1922. doi: 10.1039/d3ay00093a. Anal Methods. 2023. PMID: 37000537
-
Colorimetric Reverse Transcription-Loop-Mediated Isothermal Amplification Assay for Rapid Detection of SARS-CoV-2.Am J Trop Med Hyg. 2021 Jun 15;105(2):375-377. doi: 10.4269/ajtmh.21-0150. Am J Trop Med Hyg. 2021. PMID: 34129521 Free PMC article.
Cited by
-
Advances in ligase-based nucleic acid amplification technology for detecting gene mutations: a review.Mol Cell Biochem. 2023 Jul;478(7):1621-1631. doi: 10.1007/s11010-022-04615-w. Epub 2022 Nov 28. Mol Cell Biochem. 2023. PMID: 36441353 Free PMC article. Review.
-
Conventional and Microfluidic Methods for the Detection of Nucleic Acid of SARS-CoV-2.Micromachines (Basel). 2022 Apr 17;13(4):636. doi: 10.3390/mi13040636. Micromachines (Basel). 2022. PMID: 35457940 Free PMC article. Review.
-
Fluorescence spectrophotometry for COVID-19 determination in clinical swab samples.Arab J Chem. 2022 Aug;15(8):104020. doi: 10.1016/j.arabjc.2022.104020. Epub 2022 May 30. Arab J Chem. 2022. PMID: 35664893 Free PMC article.
-
Aptamers-Diagnostic and Therapeutic Solution in SARS-CoV-2.Int J Mol Sci. 2022 Jan 26;23(3):1412. doi: 10.3390/ijms23031412. Int J Mol Sci. 2022. PMID: 35163338 Free PMC article. Review.
-
Oligonucleotide aptamers for pathogen detection and infectious disease control.Theranostics. 2021 Aug 27;11(18):9133-9161. doi: 10.7150/thno.61804. eCollection 2021. Theranostics. 2021. PMID: 34522231 Free PMC article. Review.
References
-
- Rajapaksha P, et al. A review of methods for the detection of pathogenic microorganisms. Analyst. 2019;144:396–411. - PubMed
-
- Sintchenko V, Gallego B. Laboratory-guided detection of disease outbreaks: three generations of surveillance systems. Arch. Pathol. Lab. Med. 2009;133:916–925. - PubMed
-
- O’Connor L, Glynn B. Recent advances in the development of nucleic acid diagnostics. Expert Rev. Med. Devices. 2010;7:529–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- No. 2019R1A2C2008680/National Research Foundation of Korea (NRF)/International
- NRF-2018M3D3A1A01055754/National Research Foundation of Korea (NRF)/International
- No. 2018R1C1B3007409/National Research Foundation of Korea (NRF)/International
- No. 20194030202330/Ministry of Knowledge Economy | Korea Institute of Energy Technology Evaluation and Planning (KETEP)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

