Neurotrophin Signaling in Medulloblastoma
- PMID: 32906676
- PMCID: PMC7564905
- DOI: 10.3390/cancers12092542
Neurotrophin Signaling in Medulloblastoma
Abstract
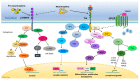
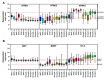
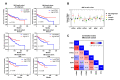
Neurotrophins are a family of secreted proteins that act by binding to tropomyosin receptor kinase (Trk) or p75NTR receptors to regulate nervous system development and plasticity. Increasing evidence indicates that neurotrophins and their receptors in cancer cells play a role in tumor growth and resistance to treatment. In this review, we summarize evidence indicating that neurotrophin signaling influences medulloblastoma (MB), the most common type of malignant brain cancer afflicting children. We discuss the potential of neurotrophin receptors as new therapeutic targets for the treatment of MB. Overall, activation of TrkA and TrkC types of receptors seem to promote cell death, whereas TrkB might stimulate MB growth, and TrkB inhibition displays antitumor effects. Importantly, we show analyses of the gene expression profile of neurotrophins and their receptors in MB primary tumors, which indicate, among other findings, that higher levels of NTRK1 or NTRK2 are associated with reduced overall survival (OS) of patients with SHH MB tumors.
Keywords: brain tumor; brain-derived neurotrophic factor; medulloblastoma; nerve growth factor; neurotrophin; tropomyosin receptor kinase.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
Grants and funding
- 409287/2016-4/National Council for Scientific and Technological Development (CNPq)
- 305647/2019-9/National Council for Scientific and Technological Development (CNPq)
- 25000.162.034/2014-21/PRONON/Ministry of Health, Brazil
- Not applicable/Children's Cancer Institute (ICI)
- 17/2551-0001 071-0/Rio Grande do Sul State Research Foundation (FAPERGS)
- Not applicable/Coordination for the Improvement of Higher Education Personnel (CAPES)
- Not applicable/Brain Tumour Foundation of Canada
- Not applicable/Canadian Institutes for Health Research
- Not applicable/American Brain Tumor Association
- Not applicable/NH/NIH HHS/United States
- Not applicable/Pediatric Brain Tumor Foundation
- Not applicable/Terry Fox Research Institute
- Not applicable/CAPMC/ CIHR/Canada
- Not applicable/Cure Search Foundation
- Not applicable/b.r.a.i.n.child
- Not applicable/Meagan's Walk
- Not applicable/Genome Canada
- Not applicable/Genome British Columbia
- Not applicable/Ontario Institute for Cancer Research
- Not applicable/Canadian Cancer Society Research Institute
LinkOut - more resources
Full Text Sources
Miscellaneous

