HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT
- PMID: 17140287
- PMCID: PMC1665652
- DOI: 10.1371/journal.ppat.0020127
HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT
Abstract
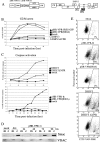
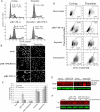
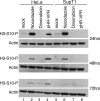
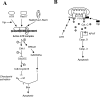
The HIV-1 accessory protein viral protein R (Vpr) causes G2 arrest and apoptosis in infected cells. We previously identified the DNA damage-signaling protein ATR as the cellular factor that mediates Vpr-induced G2 arrest and apoptosis. Here, we examine the mechanism of induction of apoptosis by Vpr and how it relates to induction of G2 arrest. We find that entry into G2 is a requirement for Vpr to induce apoptosis. We investigated the role of the mitochondrial permeability transition pore by knockdown of its essential component, the adenine nucleotide translocator. We found that Vpr-induced apoptosis was unaffected by knockdown of ANT. Instead, apoptosis is triggered through a different mitochondrial pore protein, Bax. In support of the idea that checkpoint activation and apoptosis induction are functionally linked, we show that Bax activation by Vpr was ablated when ATR or GADD45alpha was knocked down. Certain mutants of Vpr, such as R77Q and I74A, identified in long-term nonprogressors, have been proposed to inefficiently induce apoptosis while activating the G2 checkpoint in a normal manner. We tested the in vitro phenotypes of these mutants and found that their abilities to induce apoptosis and G2 arrest are indistinguishable from those of HIV-1NL4-3 vpr, providing additional support to the idea that G2 arrest and apoptosis induction are mechanistically linked.
Conflict of interest statement
Figures



References
-
- Derdeyn CA, Silvestri G. Viral and host factors in the pathogenesis of HIV infection. Curr Opin Immunol. 2005;17:366–373. - PubMed
-
- Gougeon ML. Apoptosis as an HIV strategy to escape immune attack. Nat Rev Immunol. 2003;3:392–404. - PubMed
-
- Roshal M, Zhu Y, Planelles V. Apoptosis in AIDS. Apoptosis. 2001;6:103–116. - PubMed
-
- Haase AT. Population biology of HIV-1 infection: Viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. - PubMed
-
- Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: Virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

