Isolation, cloning, and expression of an acid phosphatase containing phosphotyrosyl phosphatase activity from Prevotella intermedia
- PMID: 10559178
- PMCID: PMC94187
- DOI: 10.1128/JB.181.22.7107-7114.1999
Isolation, cloning, and expression of an acid phosphatase containing phosphotyrosyl phosphatase activity from Prevotella intermedia
Abstract
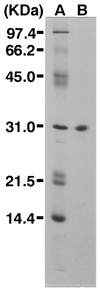
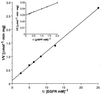
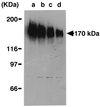
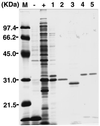
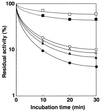
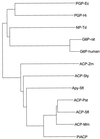
A novel acid phosphatase containing phosphotyrosyl phosphatase (PTPase) activity, designated PiACP, from Prevotella intermedia ATCC 25611, an anaerobe implicated in progressive periodontal disease, has been purified and characterized. PiACP, a monomer with an apparent molecular mass of 30 kDa, did not require divalent metal cations for activity and was sensitive to orthovanadate but highly resistant to okadaic acid. The enzyme exhibited substantial activity against tyrosine phosphate-containing peptides derived from the epidermal growth factor receptor. On the basis of N-terminal and internal amino acid sequences of purified PiACP, the gene coding for PiACP was isolated and sequenced. The PiACP gene consisted of 792 bp and coded for a basic protein with an M(r) of 29,164. The deduced amino acid sequence exhibited striking similarity (25 to 64%) to those of members of class A bacterial acid phosphatases, including PhoC of Morganella morganii, and involved a conserved phosphatase sequence motif that is shared among several lipid phosphatases and the mammalian glucose-6-phosphatases. The highly conservative motif HCXAGXXR in the active domain of PTPase was not found in PiACP. Mutagenesis of recombinant PiACP showed that His-170 and His-209 were essential for activity. Thus, the class A bacterial acid phosphatases including PiACP may function as atypical PTPases, the biological functions of which remain to be determined.
Figures


References
-
- Ansai T, Yamashita Y, Awano S, Shibata Y, Wachi M, Nagai K, Takehara T. A murC gene in Porphyromonas gingivalis 381. Microbiology. 1995;141:2047–2052. - PubMed
-
- Ansai T, Dupuy L C, Barik S. Interactions between a minimal protein serine/threonine phosphatase and its phosphopeptide substrate sequence. J Biol Chem. 1996;271:24401–24407. - PubMed
-
- Ansai T, Awano S, Chen X C, Fuchi T, Arimoto T, Akifusa S, Takehara T. Purification and characterization of alkaline phosphatase containing phosphotyrosyl phosphatase activity from the bacterium Prevotella intermedia. FEBS Lett. 1998;428:157–160. - PubMed
-
- Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996;11:266–273. - PubMed
-
- Barik S. Site-directed mutagenesis by PCR; substitution, insertion, deletion, and gene fusion. Methods Neurosci. 1995;26:309–323.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

