Functional development of Src tyrosine kinases during evolution from a unicellular ancestor to multicellular animals
- PMID: 16873552
- PMCID: PMC1567691
- DOI: 10.1073/pnas.0600021103
Functional development of Src tyrosine kinases during evolution from a unicellular ancestor to multicellular animals
Abstract
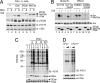
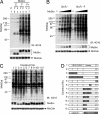
The Src family of tyrosine kinases play pivotal roles in regulating cellular functions characteristic of multicellular animals, including cell-cell interactions, cell-substrate adhesion, and cell migration. To investigate the functional alteration of Src kinases during evolution from a unicellular ancestor to multicellular animals, we characterized Src orthologs from the unicellular choanoflagellate Monosiga ovata and the primitive multicellular sponge Ephydatia fluviatilis. Here, we show that the src gene family and its C-terminal Src kinase (Csk)-mediated regulatory system already were established in the unicellular M. ovata and that unicellular Src has unique features relative to multicellular Src: It can be phosphorylated by Csk at the negative regulatory site but still exhibits substantial activity even in the phosphorylated form. Analyses of chimera molecules between M. ovata and E. fluviatilis Src orthologs reveal that structural alterations in the kinase domain are responsible for the unstable negative regulation of M. ovata Src. When expressed in vertebrate fibroblasts, M. ovata Src can induce cell transformation irrespective of the presence of Csk. These findings suggest that a structure of Src required for the stable Csk-mediated negative regulation still is immature in the unicellular M. ovata and that the development of stable negative regulation of Src may correlate with the evolution of multicellularity in animals.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

