Overexpression, purification and characterization of RecJ protein from Thermus thermophilus HB8 and its core domain
- PMID: 11713311
- PMCID: PMC92510
- DOI: 10.1093/nar/29.22.4617
Overexpression, purification and characterization of RecJ protein from Thermus thermophilus HB8 and its core domain
Abstract
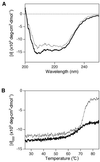
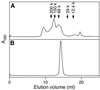
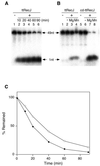
A recJ homolog was cloned from the extremely thermophilic bacterium Thermus themophilus HB8. It encodes a 527 amino acid protein that has 33% identity to Escherichia coli RecJ protein and includes the characteristic motifs conserved among RecJ homologs. Although T.thermophilus RecJ protein (ttRecJ) was expressed as an inclusion body, it was purified in soluble form through denaturation with urea and subsequent refolding steps. Limited proteolysis showed that ttRecJ has a protease-resistant core domain, which includes all the conserved motifs. We constructed a truncated ttRecJ gene that corresponds to the core domain (cd-ttRecJ). cd-ttRecJ was overexpressed in soluble form and purified. ttRecJ and cd-ttRecJ were stable up to 60 degrees C. Size exclusion chromatography indicated that ttRecJ exists in several oligomeric states, whereas cd-ttRecJ is monomeric in solution. Both proteins have 5'-->3' exonuclease activity, which was enhanced by increasing the temperature to 50 degrees C. Mg(2+), Mn(2+) or Co(2+) ions were required to activate both proteins, whereas Ca(2+) and Zn(2+) had no effects.
Figures



References
-
- Friedberg E.C., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis. American Society of Microbiology, Washington, DC.
-
- Kowalczykowski S.C. (2000) Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci., 25, 156–165. - PubMed
-
- Cooper D.L., Lahue,R.S. and Modrich,P. (1993) Methyl-directed mismatch repair is bidirectional. J. Biol. Chem., 268, 11823–11829. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

