Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism
- PMID: 15502868
- PMCID: PMC521171
- DOI: 10.1371/journal.pbio.0020327
Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism
Abstract
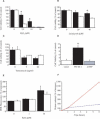
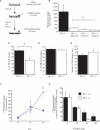
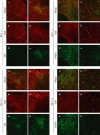
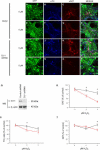
The hallmark of Parkinson's disease (PD) is the selective loss of dopamine neurons in the ventral midbrain. Although the cause of neurodegeneration in PD is unknown, a Mendelian inheritance pattern is observed in rare cases, indicating a genetic factor. Furthermore, pathological analyses of PD substantia nigra have correlated cellular oxidative stress and altered proteasomal function with PD. Homozygous mutations in DJ-1 were recently described in two families with autosomal recessive Parkinsonism, one of which is a large deletion that is likely to lead to loss of function. Here we show that embryonic stem cells deficient in DJ-1 display increased sensitivity to oxidative stress and proteasomal inhibition. The accumulation of reactive oxygen species in toxin-treated DJ-1-deficient cells initially appears normal, but these cells are unable to cope with the consequent damage that ultimately leads to apoptotic death. Furthermore, we find that dopamine neurons derived from in vitro-differentiated DJ-1-deficient embryonic stem cells display decreased survival and increased sensitivity to oxidative stress. These data are consistent with a protective role for DJ-1, and demonstrate the utility of genetically modified embryonic stem cell-derived neurons as cellular models of neuronal disorders.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures

References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. - PubMed
-
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
-
- Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

