Engineering the unicellular alga Phaeodactylum tricornutum for high-value plant triterpenoid production
- PMID: 29754445
- PMCID: PMC6330534
- DOI: 10.1111/pbi.12948
Engineering the unicellular alga Phaeodactylum tricornutum for high-value plant triterpenoid production
Abstract
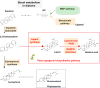
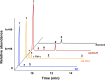
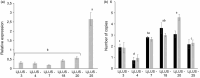
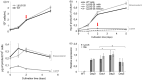
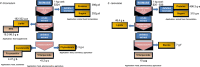
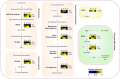
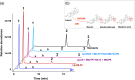
Plant triterpenoids constitute a diverse class of organic compounds that play a major role in development, plant defence and environmental interaction. Several triterpenes have demonstrated potential as pharmaceuticals. One example is betulin, which has shown promise as a pharmaceutical precursor for the treatment of certain cancers and HIV. Major challenges for triterpenoid commercialization include their low production levels and their cost-effective purification from the complex mixtures present in their natural hosts. Therefore, attempts to produce these compounds in industrially relevant microbial systems such as bacteria and yeasts have attracted great interest. Here, we report the production of the triterpenes betulin and its precursor lupeol in the photosynthetic diatom Phaeodactylum tricornutum, a unicellular eukaryotic alga. This was achieved by introducing three plant enzymes in the microalga: a Lotus japonicus oxidosqualene cyclase and a Medicago truncatula cytochrome P450 along with its native reductase. The introduction of the L. japonicus oxidosqualene cyclase perturbed the mRNA expression levels of the native mevalonate and sterol biosynthesis pathway. The best performing strains were selected and grown in a 550-L pilot-scale photobioreactor facility. To our knowledge, this is the most extensive pathway engineering undertaken in a diatom and the first time that a sapogenin has been artificially produced in a microalga, demonstrating the feasibility of the photo-bio-production of more complex high-value, metabolites in microalgae.
Keywords: algal synthetic biology; betulin; blue biotechnology; diatoms; lupeol; microalgae; natural product; triterpenoid biosynthesis.
© 2018 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
At the time of the study, SD, JS, GS, GPL, APS and MM were employed by Algenuity, a division of Spicer Consulting Limited, UK, which manufactures the Algem photobioreactor used for parts of this study.
Figures
References
-
- Anterola, A. , Shanle, E. , Perroud, P.F. and Quatrano, R. (2009) Production of taxa‐4 (5), 11 (12)‐diene by transgenic Physcomitrella patens . Transgenic Res. 18, 655–660. - PubMed
-
- Apt, K.E. , Kroth‐Pancic, P.G. and Grossman, A.R. (1996) Stable nuclear transformation of the diatom Phaeodactylum tricornutum . Mol. Gen. Genet. 252, 572–579. - PubMed
-
- Asadollahi, M.A. , Maury, J. , Møller, K. , Nielsen, K.F. , Schalk, M. , Clark, A. and Nielsen, J. (2008) Production of plant sesquiterpenes in Saccharomyces cerevisiae: effect of ERG9 repression on sesquiterpene biosynthesis. Biotechnol. Bioeng. 99, 666–677. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

