OsYSL18 is a rice iron(III)-deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints
- PMID: 19468840
- PMCID: PMC2706380
- DOI: 10.1007/s11103-009-9500-3
OsYSL18 is a rice iron(III)-deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints
Abstract
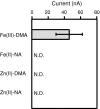
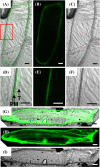
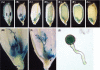
Iron uptake and translocation in plants are important processes for both plant and human nutrition, whereas relatively little is known about the molecular mechanisms of iron transport within the plant body. Several reports have shown that yellow stripe 1 (YS1) and YS1-like (YSL) transporters mediate metal-phytosiderophore uptake and/or metal-nicotianamine translocation. Among the 18 YSL genes in rice (OsYSLs), OsYSL18 is predicted to encode a polypeptide of 679 amino acids containing 13 putative transmembrane domains. An OsYSL18-green fluorescent protein (GFP) fusion was localized to the plasma membrane when transiently expressed in onion epidermal cells. Electrophysiological measurements using Xenopus laevis oocytes showed that OsYSL18 transports iron(III)-deoxymugineic acid, but not iron(II)-nicotianamine, zinc(II)-deoxymugineic acid, or zinc(II)-nicotianamine. Reverse transcriptase PCR analysis revealed more OsYSL18 transcripts in flowers than in shoots or roots. OsYSL18 promoter-beta-glucuronidase (GUS) analysis revealed that OsYSL18 was expressed in reproductive organs including the pollen tube. In vegetative organs, OsYSL18 was specifically expressed in lamina joints, the inner cortex of crown roots, and phloem parenchyma and companion cells at the basal part of every leaf sheath. These results suggest that OsYSL18 is an iron-phytosiderophore transporter involved in the translocation of iron in reproductive organs and phloem in joints.
Figures


References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.M604133200', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.m604133200'}, {'type': 'PubMed', 'value': '16926158', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16926158/'}]}
- Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 281:32395–32402. doi:10.1074/jbc.M604133200 - PubMed
-
- None
- Benes I, Schreiber K, Ripperger H, Kircheiss A (1983) Metal complex formation by nicotianamine, a possible phytosiderophore. Experientia 39:261–262. doi:10.1007/BF01955293
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s004250100552', 'is_inner': False, 'url': 'https://doi.org/10.1007/s004250100552'}, {'type': 'PubMed', 'value': '11678274', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11678274/'}]}
- Bughio N, Nakanishi H, Kiyomiya S, Matsuhashi S, Ishioka N, Watanabe S, Uchida H, Tsuji A, Osa A, Kume T, Hashimoto S, Sekine T, Mori S (2001) Real-time [11C]methionine translocation in barley in relation to mugineic acid family phytosiderophores. Planta 213:708–715. doi:10.1007/s004250100552 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/35053080', 'is_inner': False, 'url': 'https://doi.org/10.1038/35053080'}, {'type': 'PubMed', 'value': '11201743', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11201743/'}]}
- Curie C, Panavience Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL (2001) Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409:346–349. doi:10.1038/35053080 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1093/aob/mcn207', 'is_inner': False, 'url': 'https://doi.org/10.1093/aob/mcn207'}, {'type': 'PMC', 'value': 'PMC2707284', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2707284/'}, {'type': 'PubMed', 'value': '18977764', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/18977764/'}]}
- Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S (2008) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot (Lond) 103:1–11. doi:10.1093/aob/mcn207 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

