Bispecific abs against modified protein and DNA with oxidized lipids
- PMID: 16603628
- PMCID: PMC1458848
- DOI: 10.1073/pnas.0600865103
Bispecific abs against modified protein and DNA with oxidized lipids
Abstract
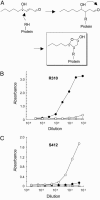
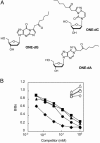
4-Hydroxy-2-nonenal (HNE), a racemic mixture of 4R- and 4S-enantiomers, is a major product of lipid peroxidation and is believed to be largely responsible for the cytopathological effects observed during oxidative stress. HNE reacts with histidine to form a stable HNE-histidine Michael addition-type adduct possessing three chiral centers in the cyclic hemiacetal structure. We have previously raised the mAbs, anti-R mAb 310 and anti-S mAb S412, that enantioselectively recognized the R-HNE-histidine and R-HNE-histidine adducts, respectively, and demonstrated the presence of both epitopes in vivo. In the present study, to further investigate the anti-HNE immune response, we analyzed the variable genes and primary structure of these Abs and found that the sequence of R310 was highly homologous to anti-DNA autoantibodies, the hallmark of systemic lupus erythematosus. An x-ray crystallographic analysis of the R310 Fab fragment showed that the R-HNE-histidine adduct binds to a hydrophobic pocket in the antigen-binding site. Despite the structural identity to the anti-DNA autoantibodies, however, R310 showed only a slight crossreactivity with the native double-stranded DNA, whereas the Ab immunoreactivity was dramatically enhanced by the treatment of the DNA with 4-oxo-2-nonenal (ONE), an analog of HNE. Moreover, the 7-(2-oxo-heptyl)-substituted 1,N2-etheno-type ONE-2'-deoxynucleoside adducts were identified as alternative epitopes of R310. Molecular mimicry between the R-HNE-histidine configurational isomers and the ONE-DNA base adducts is proposed for the dual crossreactivity.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
-
- Stadtman E. R., Levine R. L. Ann. N.Y. Acad. Sci. 2000;899:191–208. - PubMed
-
- Esterbauer H., Schaur R. J., Zollner H. Free Radical Biol. Med. 1991;11:81–128. - PubMed
-
- Uchida K. Free Radical Biol. Med. 2000;28:1685–1696. - PubMed
-
- Uchida K. Prog. Lipid Res. 2003;42:318–343. - PubMed
-
- Hashimoto M., Shibata T., Wasada H., Toyokuni S., Uchida K. J. Biol. Chem. 2003;278:5044–5051. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

