Embryonic thermosensitive TRPA1 determines transgenerational diapause phenotype of the silkworm, Bombyx mori
- PMID: 24639527
- PMCID: PMC3977306
- DOI: 10.1073/pnas.1322134111
Embryonic thermosensitive TRPA1 determines transgenerational diapause phenotype of the silkworm, Bombyx mori
Abstract
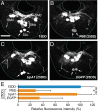
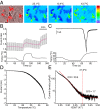
In the bivoltine strain of the silkworm, Bombyx mori, embryonic diapause is induced transgenerationally as a maternal effect. Progeny diapause is determined by the environmental temperature during embryonic development of the mother; however, its molecular mechanisms are largely unknown. Here, we show that the Bombyx TRPA1 ortholog (BmTrpA1) acts as a thermosensitive transient receptor potential (TRP) channel that is activated at temperatures above ∼ 21 °C and affects the induction of diapause in progeny. In addition, we show that embryonic RNAi of BmTrpA1 affects diapause hormone release during pupal-adult development. This study identifying a thermosensitive TRP channel that acts as a molecular switch for a relatively long-term predictive adaptive response by inducing an alternative phenotype to seasonal polyphenism is unique.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford Univ Press; 2003.
-
- Gilbert SF, Epel D. How agents in the environment effect molecular changes in development. In: Gilbert SF, Epel D, editors. Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution. Sunderland, MA: Sinauer Associates; 2009. pp. 38–78.
-
- Mousseau TA, Fox CW. Maternal Effects as Adaptations. New York: Oxford Univ Press; 1998.
-
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20(10):527–533. - PubMed
-
- Kostál V. Eco-physiological phases of insect diapause. J Insect Physiol. 2006;52(2):113–127. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

