Biochemical characterization of a novel indole prenyltransferase from Streptomyces sp. SN-593
- PMID: 20348259
- PMCID: PMC2876496
- DOI: 10.1128/JB.01557-09
Biochemical characterization of a novel indole prenyltransferase from Streptomyces sp. SN-593
Abstract
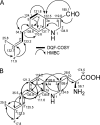
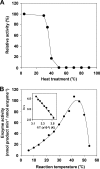
Genome sequencing of Streptomyces species has highlighted numerous potential genes of secondary metabolite biosynthesis. The mining of cryptic genes is important for exploring chemical diversity. Here we report the metabolite-guided genome mining and functional characterization of a cryptic gene by biochemical studies. Based on systematic purification of metabolites from Streptomyces sp. SN-593, we isolated a novel compound, 6-dimethylallylindole (DMAI)-3-carbaldehyde. Although many 6-DMAI compounds have been isolated from a variety of organisms, an enzyme catalyzing the transfer of a dimethylallyl group to the C-6 indole ring has not been reported so far. A homology search using known prenyltransferase sequences against the draft sequence of the Streptomyces sp. SN-593 genome revealed the iptA gene. The IptA protein showed 27% amino acid identity to cyanobacterial LtxC, which catalyzes the transfer of a geranyl group to (-)-indolactam V. A BLAST search against IptA revealed much-more-similar homologs at the amino acid level than LtxC, namely, SAML0654 (60%) from Streptomyces ambofaciens ATCC 23877 and SCO7467 (58%) from S. coelicolor A3(2). Phylogenetic analysis showed that IptA was distinct from bacterial aromatic prenyltransferases and fungal indole prenyltransferases. Detailed kinetic analyses of IptA showed the highest catalytic efficiency (6.13 min(-1) microM(-1)) for L-Trp in the presence of dimethylallyl pyrophosphate (DMAPP), suggesting that the enzyme is a 6-dimethylallyl-L-Trp synthase (6-DMATS). Substrate specificity analyses of IptA revealed promiscuity for indole derivatives, and its reaction products were identified as novel 6-DMAI compounds. Moreover, DeltaiptA mutants abolished the production of 6-DMAI-3-carbaldehyde as well as 6-dimethylallyl-L-Trp, suggesting that the iptA gene is involved in the production of 6-DMAI-3-carbaldehyde.
Figures






References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Archenbach, H., C. Renner, and R. Waibel. 1995. The hexalobines, diprenylated indoles from Hexalobus crispiflorus and Hexalobus monopetalus. Liebigs Ann. 1995:1327-1337.
-
- Archenbach, H., and B. Raffelsberger. 1979. 3,6-Bis(γ,γ-dimethylallyl)-indole from Uvaria elliotiana. Tetrahedron Lett. 28:2571-2574.
-
- Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. - PubMed
-
- Bergmann, S., J. Schumann, K. Scherlach, C. Lange, A. A. Brakhage, and C. Hertweck. 2007. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 3:213-217. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

