Cloning and structure-function analyses of quinolone- and acridone-producing novel type III polyketide synthases from Citrus microcarpa
- PMID: 23963450
- PMCID: PMC3789980
- DOI: 10.1074/jbc.M113.493155
Cloning and structure-function analyses of quinolone- and acridone-producing novel type III polyketide synthases from Citrus microcarpa
Abstract
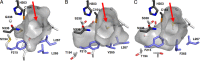
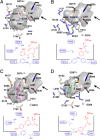
Two novel type III polyketide synthases, quinolone synthase (QNS) and acridone synthase (ACS), were cloned from Citrus microcarpa (Rutaceae). The deduced amino acid sequence of C. microcarpa QNS is unique, and it shared only 56-60% identities with C. microcarpa ACS, Medicago sativa chalcone synthase (CHS), and the previously reported Aegle marmelos QNS. In contrast to the quinolone- and acridone-producing A. marmelos QNS, C. microcarpa QNS produces 4-hydroxy-N-methylquinolone as the "single product" by the one-step condensation of N-methylanthraniloyl-CoA and malonyl-CoA. However, C. microcarpa ACS shows broad substrate specificities and produces not only acridone and quinolone but also chalcone, benzophenone, and phloroglucinol from 4-coumaroyl-CoA, benzoyl-CoA, and hexanoyl-CoA, respectively. Furthermore, the x-ray crystal structures of C. microcarpa QNS and ACS, solved at 2.47- and 2.35-Å resolutions, respectively, revealed wide active site entrances in both enzymes. The wide active site entrances thus provide sufficient space to facilitate the binding of the bulky N-methylanthraniloyl-CoA within the catalytic centers. However, the active site cavity volume of C. microcarpa ACS (760 Å(3)) is almost as large as that of M. sativa CHS (750 Å(3)), and ACS produces acridone by employing an active site cavity and catalytic machinery similar to those of CHS. In contrast, the cavity of C. microcarpa QNS (290 Å(3)) is significantly smaller, which makes this enzyme produce the diketide quinolone. These results as well as mutagenesis analyses provided the first structural bases for the anthranilate-derived production of the quinolone and acridone alkaloid by type III polyketide synthases.
Keywords: Biosynthesis; Cloning; Enzymes; Polyketides; Structural Biology.
Figures




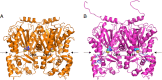

References
-
- Austin M. B., Noel J. P. (2003) The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 20, 79–110 - PubMed
-
- Abe I., Morita H. (2010) Structure and function of the chalcone synthase superfamily of plant type III polyketide syntheses. Nat. Prod. Rep. 27, 809–838 - PubMed
-
- Abe I., Watanabe T., Noguchi H. (2004) Enzymatic formation of long-chain polyketide pyrones by plant type III polyketide synthases. Phytochemistry 65, 2447–2453 - PubMed
-
- Wanibuchi K., Zhang P., Abe T., Morita H., Kohno T., Chen G., Noguchi H., Abe I. (2007) An acridone-producing novel multifunctional type III polyketide synthase from Huperzia serrata. FEBS J. 274, 1073–1082 - PubMed
-
- Wakimoto T., Mori T., Morita H., Abe I. (2011) Cytotoxic tetramic acid derivative produced by a plant type-III polyketide synthase. J. Am. Chem. Soc. 133, 4746–4749 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

