The mannose-binding lectin gene FaMBL1 is involved in the resistance of unripe strawberry fruits to Colletotrichum acutatum
- PMID: 24690196
- PMCID: PMC6638621
- DOI: 10.1111/mpp.12143
The mannose-binding lectin gene FaMBL1 is involved in the resistance of unripe strawberry fruits to Colletotrichum acutatum
Abstract
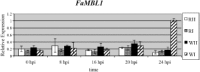
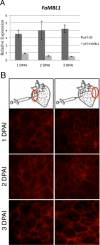
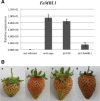
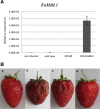
The fungal pathogen Colletotrichum acutatum is the causal agent of strawberry (Fragaria × ananassa) anthracnose. Although the fungus can infect strawberry fruits at both unripe and ripe stages, the symptoms appear only on red ripe fruits. On white unripe fruits, the pathogen becomes quiescent as melanized appressoria after 24 h of interaction. Previous transcriptome analysis has indicated that a mannose-binding lectin (MBL) gene is the most up-regulated gene in 24-h-infected white strawberries, suggesting a role for this gene in the low susceptibility of unripe stages. A time course analysis of the expression of this MBL gene, named FaMBL1 (Fragaria × ananassa MBL 1a), was undertaken to monitor its expression profile in white and red fruits at early interaction times: FaMBL1 was expressed exclusively in white fruit after 24 h, when the pathogen was quiescent. Agrobacterium-mediated transient transformation was used to silence and overexpress the FaMBL1 gene in 24-h-infected white and red strawberries, respectively. FaMBL1-silenced unripe fruits showed an increase in susceptibility to C. acutatum. These 24-h-infected tissues contained subcuticular hyphae, indicating pathogen penetration and active growth. In contrast, overexpression of FaMBL1 in ripe fruits decreased susceptibility; here, 24-h-infected tissues showed a high percentage of ungerminated appressoria, suggesting that the growth of the pathogen had slowed. These data suggest that FaMBL1 plays a crucial role in the resistance of unripe strawberry fruits to C. acutatum.
Keywords: Colletotrichum acutatum; fungal quiescence; mannose binding lectin; strawberry ripening; unripe fruit resistance.
© 2014 BSPP AND JOHN WILEY & SONS LTD.
Figures


References
-
- Araujo‐Filho, J.H. , Vasconcelos, I.M. , Martins‐Miranda, A.S. , Gondim, D.M. and Oliveira, J.T. (2010) A ConA‐like lectin from Dioclea guianensis Benth. has antifungal activity against Colletotrichum gloeosporioides, unlike its homologues, ConM and ConA. J. Agric. Food Chem. 58, 4090–4096. - PubMed
-
- Bailey, J.A. and Jeger, M.J. (1992) Colletotrichum: Biology, Pathology and Control. Wallingford, Oxfordshire: CAB International.
-
- Bailey, J.A. , O'Connel, R.J. , Pring, R.J. and Nash, C. (1992) Infection strategies of Colletotrichum species In: Colletotrichum: Biology, Pathology and Control (Bailey J.A. and Jeger M.J., eds), pp. 88–120. Wallingford, Oxfordshire: CAB International.
-
- Barre, A. , Bourne, Y. , Van Damme, E.J.M. , Peumans, W.J. and Rougé, P. (2001) Mannose‐binding plant lectins: different structural scaffolds for a common sugar‐recognition process. Biochimie, 83, 645–651. - PubMed
-
- Barre, A. , Herve, C. , Lescure, B. and Rougé, P. (2002) Lectin receptor kinases in plants. Crit. Rev. Plant Sci. 21, 379–399.
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

