A novel mammalian, mitotic spindle-associated kinase is related to yeast and fly chromosome segregation regulators
- PMID: 9245792
- PMCID: PMC2141637
- DOI: 10.1083/jcb.138.3.643
A novel mammalian, mitotic spindle-associated kinase is related to yeast and fly chromosome segregation regulators
Abstract
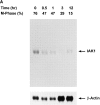
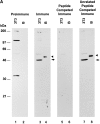
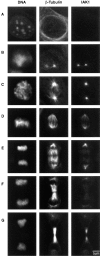
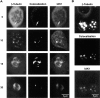
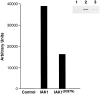
We describe a novel mammalian protein kinase related to two newly identified yeast and fly kinases-Ipl1 and aurora, respectively-mutations in which cause disruption of chromosome segregation. We have designated this kinase as Ipl1- and aurora-related kinase 1 (IAK1). IAK1 expression in mouse fibroblasts is tightly regulated temporally and spatially during the cell cycle. Transcripts first appear at G1/S boundary, are elevated at M-phase, and disappear rapidly after completion of mitosis. The protein levels and kinase activity of IAK1 are also cell cycle regulated with a peak at M-phase. IAK1 protein has a distinct subcellular and temporal pattern of localization. It is first identified on the centrosomes immediately after the duplicated centrosomes have separated. The protein remains on the centrosome and the centrosome-proximal part of the spindle throughout mitosis and is detected weakly on midbody microtubules at telophase and cytokinesis. In cells recovering from nocodazole treatment and in taxol-treated mitotic cells, IAK1 is associated with microtubule organizing centers. A wild-type and a mutant form of IAK1 cause mitotic spindle defects and lethality in ipl1 mutant yeast cells but not in wild-type cells, suggesting that IAK1 interferes with Ipl1p function in yeast. Taken together, these data strongly suggest that IAK1 may have an important role in centrosome and/ or spindle function during chromosome segregation in mammalian cells. We suggest that IAK1 is a new member of an emerging subfamily of the serine/threonine kinase superfamily. The members of this subfamily may be important regulators of chromosome segregation.
Figures








References
-
- Altschul, S.F., W. Gish, W. Miller, E.W. Myers, and D.J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol., 215:403–410. - PubMed
-
- Blangy A, Lane HA, d'Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

