A prevalent variant in PPP1R3A impairs glycogen synthesis and reduces muscle glycogen content in humans and mice
- PMID: 18232732
- PMCID: PMC2214798
- DOI: 10.1371/journal.pmed.0050027
A prevalent variant in PPP1R3A impairs glycogen synthesis and reduces muscle glycogen content in humans and mice
Erratum in
- PLoS Med. 2008 Dec;5(12):e246.. Solanky, Bhavana [added]; Deelchand, Dinesh [added]
Abstract
Background: Stored glycogen is an important source of energy for skeletal muscle. Human genetic disorders primarily affecting skeletal muscle glycogen turnover are well-recognised, but rare. We previously reported that a frameshift/premature stop mutation in PPP1R3A, the gene encoding RGL, a key regulator of muscle glycogen metabolism, was present in 1.36% of participants from a population of white individuals in the UK. However, the functional implications of the mutation were not known. The objective of this study was to characterise the molecular and physiological consequences of this genetic variant.
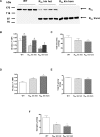
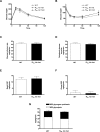
Methods and findings: In this study we found a similar prevalence of the variant in an independent UK white population of 744 participants (1.46%) and, using in vivo (13)C magnetic resonance spectroscopy studies, demonstrate that human carriers (n = 6) of the variant have low basal (65% lower, p = 0.002) and postprandial muscle glycogen levels. Mice engineered to express the equivalent mutation had similarly decreased muscle glycogen levels (40% lower in heterozygous knock-in mice, p < 0.05). In muscle tissue from these mice, failure of the truncated mutant to bind glycogen and colocalize with glycogen synthase (GS) decreased GS and increased glycogen phosphorylase activity states, which account for the decreased glycogen content.
Conclusions: Thus, PPP1R3A C1984DeltaAG (stop codon 668) is, to our knowledge, the first prevalent mutation described that directly impairs glycogen synthesis and decreases glycogen levels in human skeletal muscle. The fact that it is present in approximately 1 in 70 UK whites increases the potential biomedical relevance of these observations.
Conflict of interest statement
Figures




Comment in
-
New insights into impaired muscle glycogen synthesis.PLoS Med. 2008 Jan 29;5(1):e25. doi: 10.1371/journal.pmed.0050025. PLoS Med. 2008. PMID: 18232730 Free PMC article.
References
-
- Savage DB, Agostini M, Barroso I, Gurnell M, Luan J, et al. Digenic inheritance of severe insulin resistance in a human pedigree. Nat Genet. 2002;31:379–384. - PubMed
-
- Lanner C, Suzuki Y, Bi C, Zhang H, Cooper LD, et al. Gene structure and expression of the targeting subunit, RGL, of the muscle-specific glycogen-associated type 1 protein phosphatase, PP1G. Arch Biochem Biophys. 2001;388:135–145. - PubMed
-
- Hubbard MJ, Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993;18:172–177. - PubMed
-
- Tang PM, Bondor JA, Swiderek KM, DePaoli-Roach AA. Molecular cloning and expression of the regulatory (RG1) subunit of the glycogen-associated protein phosphatase. J Biol Chem. 1991;266:15782–15789. - PubMed
-
- Wu J, Kleiner U, Brautigan DL. Protein phosphatase type-1 and glycogen bind to a domain in the skeletal muscle regulatory subunit containing conserved hydrophobic sequence motif. Biochemistry. 1996;35:13858–13864. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

