Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp
- PMID: 9647833
- PMCID: PMC106429
- DOI: 10.1128/AEM.64.7.2578-2584.1998
Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp
Abstract
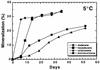
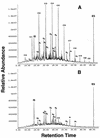
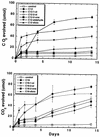
The psychorotrophic Rhodococcus sp. strain Q15 was examined for its ability to degrade individual n-alkanes and diesel fuel at low temperatures, and its alkane catabolic pathway was investigated by biochemical and genetic techniques. At 0 and 5 degrees C, Q15 mineralized the short-chain alkanes dodecane and hexadecane to a greater extent than that observed for the long-chain alkanes octacosane and dotriacontane. Q15 utilized a broad range of aliphatics (C10 to C21 alkanes, branched alkanes, and a substituted cyclohexane) present in diesel fuel at 5 degrees C. Mineralization of hexadecane at 5 degrees C was significantly greater in both hydrocarbon-contaminated and pristine soil microcosms seeded with Q15 cells than in uninoculated control soil microcosms. The detection of hexadecane and dodecane metabolic intermediates (1-hexadecanol and 2-hexadecanol and 1-dodecanol and 2-dodecanone, respectively) by solid-phase microextraction-gas chromatography-mass spectrometry and the utilization of potential metabolic intermediates indicated that Q15 oxidizes alkanes by both the terminal oxidation pathway and the subterminal oxidation pathway. Genetic characterization by PCR and nucleotide sequence analysis indicated that Q15 possesses an aliphatic aldehyde dehydrogenase gene highly homologous to the Rhodococcus erythropolis the A gene. Rhodococcus sp. strain Q15 possessed two large plasmids of approximately 90 and 115 kb (shown to mediate Cd resistance) which were not required for alkane mineralization, although the 90-kb plasmid enhanced mineralization of some alkanes and growth on diesel oil at both 5 and 25 degrees C.
Figures





References
-
- Anderson J P E. Soil respiration. In: Page A L, editor. Methods of soil analysis, part 2. Chemical and microbiological properties. 2nd ed. Madison, Wis: American Society of Agronomy; 1982. pp. 836–841.
-
- Arthur C L, Pawliszyn J. Solid-phase microextraction. Anal Chem. 1990;66:2145–2148.
-
- Bossert I, Bartha R. The fate of petroleum in soil ecosystems. In: Atlas R M, editor. Petroleum microbiology. New York, N.Y: Macmillan Publishing Co.; 1984. pp. 435–474.
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

