Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein
- PMID: 9765397
- PMCID: PMC110269
- DOI: 10.1128/JVI.72.11.8586-8596.1998
Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein
Abstract
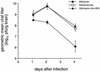
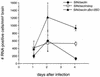
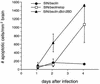
bcl-2, the prototypic cellular antiapoptotic gene, decreases Sindbis virus replication and Sindbis virus-induced apoptosis in mouse brains, resulting in protection against lethal encephalitis. To investigate potential mechanisms by which Bcl-2 protects against central nervous system Sindbis virus infection, we performed a yeast two-hybrid screen to identify Bcl-2-interacting gene products in an adult mouse brain library. We identified a novel 60-kDa coiled-coil protein, Beclin, which we confirmed interacts with Bcl-2 in mammalian cells, using fluorescence resonance energy transfer microscopy. To examine the role of Beclin in Sindbis virus pathogenesis, we constructed recombinant Sindbis virus chimeras that express full-length human Beclin (SIN/beclin), Beclin lacking the putative Bcl-2-binding domain (SIN/beclinDeltaBcl-2BD), or Beclin containing a premature stop codon near the 5' terminus (SIN/beclinstop). The survival of mice infected with SIN/beclin was significantly higher (71%) than the survival of mice infected with SIN/beclinDeltaBcl-2BD (9%) or SIN/beclinstop (7%) (P < 0.001). The brains of mice infected with SIN/beclin had fewer Sindbis virus RNA-positive cells, fewer apoptotic cells, and lower viral titers than the brains of mice infected with SIN/beclinDeltaBcl-2BD or SIN/beclinstop. These findings demonstrate that Beclin is a novel Bcl-2-interacting cellular protein that may play a role in antiviral host defense.
Figures

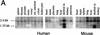
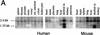


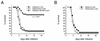

References
-
- Allsopp T E, Scallan M F, Williams A, Fazakerley J K. Virus infection induces neuronal apoptosis: a comparison with trophic factor withdrawal. Cell Death Differ. 1998;5:50–59. - PubMed
-
- Altshul S F, Gish W, Miller W, Meyers E W, Lipman D J. Basic local alignment tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nunez G, Thompson C B. Bcl-xL, a bcl-2 related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. - PubMed
-
- Cheng E H Y, Levine B, Boise L H, Thompson C B, Hardwick J M. Bax-independent inhibition of apoptosis by Bcl-xL. Nature. 1996;379:554–556. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

