Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling
- PMID: 10330411
- PMCID: PMC2133183
- DOI: 10.1083/jcb.145.4.851
Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling
Abstract
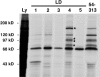
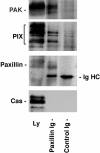
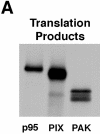
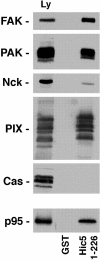
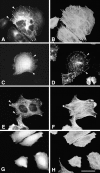
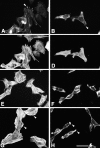
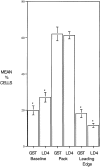
Paxillin is a focal adhesion adaptor protein involved in the integration of growth factor- and adhesion-mediated signal transduction pathways. Repeats of a leucine-rich sequence named paxillin LD motifs (Brown M.C., M.S. Curtis, and C.E. Turner. 1998. Nature Struct. Biol. 5:677-678) have been implicated in paxillin binding to focal adhesion kinase (FAK) and vinculin. Here we demonstrate that the individual paxillin LD motifs function as discrete and selective protein binding interfaces. A novel scaffolding function is described for paxillin LD4 in the binding of a complex of proteins containing active p21 GTPase-activated kinase (PAK), Nck, and the guanine nucleotide exchange factor, PIX. The association of this complex with paxillin is mediated by a new 95-kD protein, p95PKL (paxillin-kinase linker), which binds directly to paxillin LD4 and PIX. This protein complex also binds to Hic-5, suggesting a conservation of LD function across the paxillin superfamily. Cloning of p95PKL revealed a multidomain protein containing an NH2-terminal ARF-GAP domain, three ankyrin-like repeats, a potential calcium-binding EF hand, calmodulin-binding IQ motifs, a myosin homology domain, and two paxillin-binding subdomains (PBS). Green fluorescent protein- (GFP-) tagged p95PKL localized to focal adhesions/complexes in CHO.K1 cells. Overexpression in neuroblastoma cells of a paxillin LD4 deletion mutant inhibited lamellipodia formation in response to insulin-like growth fac- tor-1. Microinjection of GST-LD4 into NIH3T3 cells significantly decreased cell migration into a wound. These data implicate paxillin as a mediator of p21 GTPase-regulated actin cytoskeletal reorganization through the recruitment to nascent focal adhesion structures of an active PAK/PIX complex potentially via interactions with p95PKL.
Figures
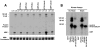









References
-
- Adam L, Vadlamuda R, Kondapaka B, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. - PubMed
-
- Ayi TC, Tsan JT, Hwang LY, Bowcock AM, Baer R. Conservation of function and primary structure in the BRCA1-associated RING domain (BARD1) protein. Oncogene. 1998;17:2143–2148. - PubMed
-
- Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA. Identification of a mouse p21Cdc42/Racactivated kinase. J Biol Chem. 1995;270:22731–22737. - PubMed
-
- Bagrodia S, Taylor SJ, Jordan KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. - PubMed
-
- Bagrodia, S., D. Bailey, Z. Leonard, M. Hart, J.-L. Guan, R.T. Premont, S.J. Taylor, and R.A. Cerione. 1999. A tyrosine phosphorylated protein that binds to an important regulatory region on the Cool family of Pak-binding proteins. J. Biol. Chem. In press. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

