Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated
- PMID: 10225900
- PMCID: PMC115983
- DOI: 10.1128/IAI.67.5.2389-2398.1999
Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated
Abstract
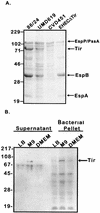
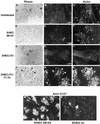
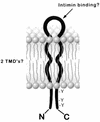
Intimate attachment to the host cell leading to the formation of attaching and effacing (A/E) lesions is an essential feature of enterohemorrhagic Escherichia coli (EHEC) O157:H7 pathogenesis. In a related pathogen, enteropathogenic E. coli (EPEC), this activity is dependent upon translocation of the intimin receptor, Tir, which becomes tyrosine phosphorylated within the host cell membrane. In contrast, the accumulation of tyrosine-phosphorylated proteins beneath adherent EHEC bacteria does not occur, leading to questions about whether EHEC uses a Tir-based mechanism for adherence and A/E lesion formation. In this report, we demonstrate that EHEC produces a functional Tir that is inserted into host cell membranes, where it serves as an intimin receptor. However, unlike in EPEC, in EHEC Tir is not tyrosine phosphorylated yet plays a key role in both bacterial adherence to epithelial cells and pedestal formation. EHEC, but not EPEC, was unable to synthesize Tir in Luria-Bertani medium but was able to secrete Tir into M9 medium, suggesting that Tir synthesis and secretion may be regulated differently in these two pathogens. EHEC Tir and EPEC Tir both bind intimin and focus cytoskeletal rearrangements, indicating that tyrosine phosphorylation is not needed for pedestal formation. EHEC and EPEC intimins are functionally interchangeable, but EHEC Tir shows a much greater affinity for EHEC intimin than for EPEC intimin. These findings highlight some of the differences and similarities between EHEC and EPEC virulence mechanisms, which can be exploited to further define the molecular basis of pedestal formation.
Figures






References
-
- Berendson R, Cheney C P, Schad P A, Boedeker E C. Species-specific binding of purified pili (AF/R1) from Escherichia coli RDEC-1 to rabbit intestinal mucosa. Gastroenterology. 1983;85:837–845. - PubMed
-
- Bieber D, Ramer S W, Wu C Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. - PubMed
-
- Brunder W, Schmidt H, Karsh H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7, cleaves human coagulation factor. V Mol Microbiol. 1997;24:767–778. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

