Toward an understanding of the biochemical and pharmacological complexity of the saliva of a hematophagous sand fly Lutzomyia longipalpis
- PMID: 10611354
- PMCID: PMC24789
- DOI: 10.1073/pnas.96.26.15155
Toward an understanding of the biochemical and pharmacological complexity of the saliva of a hematophagous sand fly Lutzomyia longipalpis
Abstract
The saliva of blood-sucking arthropods contains powerful pharmacologically active substances and may be a vaccine target against some vector-borne diseases. Subtractive cloning combined with biochemical approaches was used to discover activities in the salivary glands of the hematophagous fly Lutzomyia longipalpis. Sequences of nine full-length cDNA clones were obtained, five of which are possibly associated with blood-meal acquisition, each having cDNA similarity to: (i) the bed bug Cimex lectularius apyrase, (ii) a 5'-nucleotidase/phosphodiesterase, (iii) a hyaluronidase, (iv) a protein containing a carbohydrate-recognition domain (CRD), and (v) a RGD-containing peptide with no significant matches to known proteins in the BLAST databases. Following these findings, we observed that the salivary apyrase activity of L. longipalpis is indeed similar to that of Cimex apyrase in its metal requirements. The predicted isoelectric point of the putative apyrase matches the value found for Lutzomyia salivary apyrase. A 5'-nucleotidase, as well as hyaluronidase activity, was found in the salivary glands, and the CRD-containing cDNA matches the N-terminal sequence of the HPLC-purified salivary anticlotting protein. A cDNA similar to alpha-amylase was discovered and salivary enzymatic activity demonstrated for the first time in a blood-sucking arthropod. Full-length clones were also found coding for three proteins of unknown function matching, respectively, the N-terminal sequence of an abundant salivary protein, having similarity to the CAP superfamily of proteins and the Drosophila yellow protein. Finally, two partial sequences are reported that match possible housekeeping genes. Subtractive cloning will considerably enhance efforts to unravel the salivary pharmacopeia of blood-sucking arthropods.
Figures

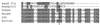
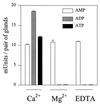



References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

