Coupling endoplasmic reticulum stress to the cell-death program: a novel HSP90-independent role for the small chaperone protein p23
- PMID: 16195741
- PMCID: PMC1847409
- DOI: 10.1038/sj.cdd.4401761
Coupling endoplasmic reticulum stress to the cell-death program: a novel HSP90-independent role for the small chaperone protein p23
Abstract
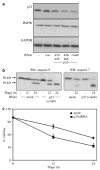
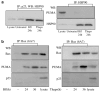
The endoplasmic reticulum (ER) is the principal organelle for the biosynthesis of proteins, steroids and many lipids, and is highly sensitive to alterations in its environment. Perturbation of Ca(2+) homeostasis, elevated secretory protein synthesis, deprivation of glucose or other sugars, altered glycosylation and/or the accumulation of misfolded proteins may all result in ER stress, and prolonged ER stress triggers cell death. Studies from multiple laboratories have identified the roles of several ER stress-induced cell-death modulators and effectors through the use of biochemical, pharmacological and genetic tools. In the present work, we describe the role of p23, a small chaperone protein, in preventing ER stress-induced cell death. p23 is a highly conserved chaperone protein that modulates HSP90 activity and is also a component of the steroid receptors. p23 is cleaved during ER stress-induced cell death; this cleavage, which occurs close to the carboxy-terminus, requires caspase-3 and/or caspase-7, but not caspase-8. Blockage of the caspase cleavage site of p23 was associated with decreased cell death induced by ER stress. Immunodepletion of p23 or inhibition of p23 expression by siRNA resulted in enhancement of ER stress-induced cell death. While p23 co-immunoprecipitated with the BH3-only protein PUMA (p53-upregulated modulator of apoptosis) in untreated cells, prolonged ER stress disrupted this interaction. The results define a protective role for p23, and provide further support for a model in which ER stress is coupled to the mitochondrial intrinsic apoptotic pathway through the activities of BH3 family proteins.
Figures





References
-
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. - PubMed
-
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. - PubMed
-
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

