Identification of an NTF2-related factor that binds Ran-GTP and regulates nuclear protein export
- PMID: 10567585
- PMCID: PMC84993
- DOI: 10.1128/MCB.19.12.8616
Identification of an NTF2-related factor that binds Ran-GTP and regulates nuclear protein export
Abstract
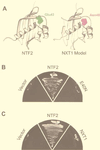
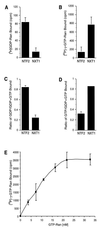
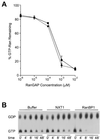
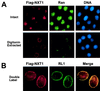
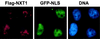
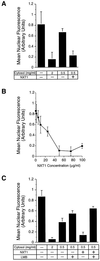
Active transport of macromolecules between the nucleus and cytoplasm requires signals for import and export and their recognition by shuttling receptors. Each class of macromolecule is thought to have a distinct receptor that mediates the transport reaction. Assembly and disassembly reactions of receptor-substrate complexes are coordinated by Ran, a GTP-binding protein whose nucleotide state is regulated catalytically by effector proteins. Ran function is modulated in a noncatalytic fashion by NTF2, a protein that mediates nuclear import of Ran-GDP. Here we characterize a novel component of the Ran system that is 26% identical to NTF2, which based on its function we refer to as NTF2-related export protein 1 (NXT1). In contrast to NTF2, NXT1 preferentially binds Ran-GTP, and it colocalizes with the nuclear pore complex (NPC) in mammalian cells. These properties, together with the fact that NXT1 shuttles between the nucleus and the cytoplasm, suggest an active role in nuclear transport. Indeed, NXT1 stimulates nuclear protein export of the NES-containing protein PKI in vitro. The export function of NXT1 is blocked by the addition of leptomycin B, a compound that selectively inhibits the NES receptor Crm1. Thus, NXT1 regulates the Crm1-dependent export pathway through its direct interaction with Ran-GTP.
Figures

References
-
- Bullock T L, Clarkson W D, Kent H M, Stewart M. 1.6 A structure of nuclear transport factor 2 (NTF2) J Mol Biol. 1996;260:422–431. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
