Divergent N-terminal sequences target an inducible testis deubiquitinating enzyme to distinct subcellular structures
- PMID: 10938131
- PMCID: PMC86134
- DOI: 10.1128/MCB.20.17.6568-6578.2000
Divergent N-terminal sequences target an inducible testis deubiquitinating enzyme to distinct subcellular structures
Abstract
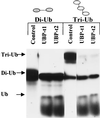
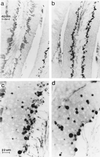
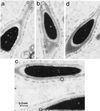
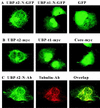
Ubiquitin-specific processing proteases (UBPs) presently form the largest enzyme family in the ubiquitin system, characterized by a core region containing conserved motifs surrounded by divergent sequences, most commonly at the N-terminal end. The functions of these divergent sequences remain unclear. We identified two isoforms of a novel testis-specific UBP, UBP-t1 and UBP-t2, which contain identical core regions but distinct N termini, thereby permitting dissection of the functions of these two regions. Both isoforms were germ cell specific and developmentally regulated. Immunocytochemistry revealed that UBP-t1 was induced in step 16 to 19 spermatids while UBP-t2 was expressed in step 18 to 19 spermatids. Immunoelectron microscopy showed that UBP-t1 was found in the nucleus while UBP-t2 was extranuclear and was found in residual bodies. For the first time, we show that the differential subcellular localization was due to the distinct N-terminal sequences. When transfected into COS-7 cells, the core region was expressed throughout the cell but the UBP-t1 and UBP-t2 isoforms were concentrated in the nucleus and the perinuclear region, respectively. Fusions of each N-terminal end with green fluorescent protein yielded the same subcellular localization as the native proteins, indicating that the N-terminal ends were sufficient for determining differential localization. Interestingly, UBP-t2 colocalized with anti-gamma-tubulin immunoreactivity, indicating that like several other components of the ubiquitin system, a deubiquitinating enzyme is associated with the centrosome. Regulated expression and alternative N termini can confer specificity of UBP function by restricting its temporal and spatial loci of action.
Figures






References
-
- Baker R T, Tobias J W, Varshavsky A. Ubiquitin-specific proteases of Saccharomyces cerevisiae. Cloning of UBP2 and UBP3, and functional analysis of the UBP gene family. J Biol Chem. 1992;267:23364–23375. - PubMed
-
- Clermont Y, Oko R, Hermo L. Cell biology of mammalian spermiogenesis. In: Desjardins C, editor. Cell and molecular biology of the testis. New York, N.Y: Oxford University Press; 1993. pp. 332–375.
-
- Coux O, Tanaka K, Goldberg A L. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
