Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis
- PMID: 10841576
- PMCID: PMC18751
- DOI: 10.1073/pnas.97.12.6820
Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis
Abstract
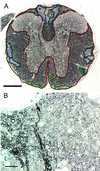
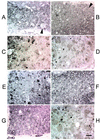
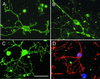
Promoting remyelination, a major goal of an effective treatment for demyelinating diseases, has the potential to protect vulnerable axons, increase conduction velocity, and improve neurologic deficits. Strategies to promote remyelination have focused on transplanting oligodendrocytes (OLs) or recruiting endogenous myelinating cells with trophic factors. Ig-based therapies, routinely used to treat a variety of neurological and autoimmune diseases, underlie our approach to enhance remyelination. We isolated two human mAbs directed against OL surface antigens that promoted significant remyelination in a virus-mediated model of multiple sclerosis. Four additional OL-binding human mAbs did not promote remyelination. Both human mAbs were as effective as human i.v. Ig, a treatment shown to have efficacy in multiple sclerosis, and bound to the surface of human OLs suggesting a direct effect of the mAbs on the cells responsible for myelination. Alternatively, targeting human mAbs to areas of central nervous system (CNS) pathology may facilitate the opsonization of myelin debris, allowing repair to proceed. Human mAbs were isolated from the sera of individuals with a form of monoclonal gammopathy. These individuals carry a high level of monoclonal protein in their blood without detriment, lending support to the belief that administration of these mAbs as a therapy would be safe. Our results are (i) consistent with the hypothesis that CNS-reactive mAbs, part of the normal Ig repertoire in humans, may help repair and protect the CNS from pathogenic immune injury, and (ii) further challenge the premise that Abs that bind OLs are necessarily pathogenic.
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

