Identification of renox, an NAD(P)H oxidase in kidney
- PMID: 10869423
- PMCID: PMC16661
- DOI: 10.1073/pnas.130135897
Identification of renox, an NAD(P)H oxidase in kidney
Abstract
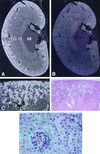
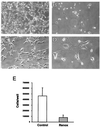
Oxygen sensing is essential for homeostasis in all aerobic organisms, but its mechanism is poorly understood. Data suggest that a phagocytic-like NAD(P)H oxidase producing reactive oxygen species serves as a primary sensor for oxygen. We have characterized a source of superoxide anions in the kidney that we refer to as a renal NAD(P)H oxidase or Renox. Renox is homologous to gp91(phox) (91-kDa subunit of the phagocyte oxidase), the electron-transporting subunit of phagocytic NADPH oxidase, and contains all of the structural motifs considered essential for binding of heme, flavin, and nucleotide. In situ RNA hybridization revealed that renox is highly expressed at the site of erythropoietin production in the renal cortex, showing the greatest accumulation of renox mRNA in proximal convoluted tubule epithelial cells. NIH 3T3 fibroblasts overexpressing transfected Renox show increased production of superoxide and develop signs of cellular senescence. Our data suggest that Renox, as a renal source of reactive oxygen species, is a likely candidate for the oxygen sensor function regulating oxygen-dependent gene expression and may also have a role in the development of inflammatory processes in the kidney.
Figures



References
-
- Leto T L. In: Inflammation Basic Principles and Clinical Correlates. Gallin J I, Snyderman R, editors. Williams & Wilkins, Philadelphia: Lippincott; 1999. pp. 769–787.
-
- Adler V, Yin Z, Tew K D, Ronai Z. Oncogene. 1999;18:6104–6111. - PubMed
-
- Ebert B L, Bunn H F. Blood. 1999;94:1864–1877. - PubMed
-
- Suh Y A, Arnold R S, Lassegue B, Shi J, Xu X, Sorescu D, Chung A B, Griendling K K, Lambeth J D. Nature (London) 1999;401:79–82. - PubMed
-
- Dupuy C, Ohayon R, Valent A, Noel-Hudson M S, Deme D, Virion A. J Biol Chem. 1999;274:37265–37269. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

