Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain
- PMID: 11016959
- PMCID: PMC17218
- DOI: 10.1073/pnas.200360997
Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain
Abstract
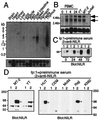
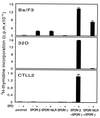
We have identified a type I cytokine receptor, which we have termed novel interleukin receptor (NILR), that is most related to the IL-2 receptor beta chain (IL-2Rbeta) and physically adjacent to the IL-4 receptor alpha chain gene on chromosome 16. NILR mRNA is most highly expressed in thymus and spleen, and is induced by phytohemagglutinin in human peripheral blood mononuclear cells. NILR protein was detected on human T cell lymphotropic virus type I-transformed T cell lines, Raji B cells, and YT natural killer-like cells. Artificial homodimerization of the NILR cytoplasmic domain confers proliferation to Ba/F3 murine pro-B cells but not to 32D myeloid progenitor cells or CTLL-2 murine helper T cells. In these latter cells, heterodimerization of IL-2Rbeta and the common cytokine receptor gamma chain (gamma(c)) cytoplasmic domains allows potent proliferation, whereas such heterodimerization of NILR with gamma(c) does not. This finding suggests that NILR has signaling potential but that a full understanding of its signaling partner(s) is not yet clear. Like IL-2Rbeta, NILR associates with Jak1 and mediates Stat5 activation.
Figures




References
-
- Leonard W J, O'Shea J J. Annu Rev Immunol. 1998;16:293–322. - PubMed
-
- Leonard W J. In: Fundamental Immunology. Paul W E, editor. Philadelphia: Lippincott; 1999. pp. 741–774.
-
- Miyajima A, Mui A L, Ogorochi T, Sakamaki K. Blood. 1993;82:1960–1974. - PubMed
-
- Leonard W J, Shores E W, Love P E. Immunol Rev. 1995;148:97–114. - PubMed
-
- Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka N, Munakata H, Nakamura M, Sugamura K. Science. 1992;257:379–382. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

