Serotonin stimulates phosphorylation of Aplysia synapsin and alters its subcellular distribution in sensory neurons
- PMID: 12097493
- PMCID: PMC6758201
- DOI: 10.1523/JNEUROSCI.22-13-05412.2002
Serotonin stimulates phosphorylation of Aplysia synapsin and alters its subcellular distribution in sensory neurons
Abstract
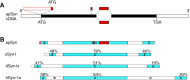
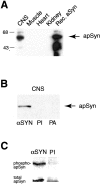
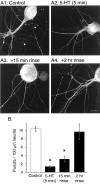
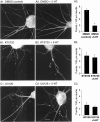
Only a small fraction of neurotransmitter-containing synaptic vesicles (SVs), the readily releasable pool, is available for fast Ca(2+)-induced release at any synapse. Most SVs are sequestered at sites away from the plasma membrane and cannot be exocytosed directly. Recruitment of SVs to the releasable pool is thought to be an important component of short-term synaptic facilitation by serotonin (5-HT) at Aplysia sensorimotor synapses. Synapsins are associated with SVs and hypothesized to play a central role in the regulation of SV mobilization in nerve terminals. Aplysia synapsin was cloned to examine its role in synaptic plasticity at the well characterized sensorimotor neuron synapse of this animal. Acute 5-HT treatment of ganglia induced synapsin phosphorylation. Immunohistochemical analyses of cultured Aplysia neurons revealed that synapsin is distributed in distinct puncta in the neurites. These puncta are rapidly dispersed after treatment of the neurons with 5-HT. The dispersion of synapsin puncta by 5-HT was fully reversible after washout of the modulator. Both 5-HT-induced phosphorylation and dispersion of synapsin were mediated, at least in part, by cAMP-dependent protein kinase and mitogen-activated protein kinase. These experiments indicate that synapsin and its regulation by 5-HT may play an important role in the modulation of SV trafficking in short-term synaptic plasticity.
Figures





References
-
- Angers A, Bean AJ, Byrne JH. Cloning and molecular characterization of Aplysia synaptic vesicle protein synapsin. Soc Neurosci Abstr. 1999;25:1749.
-
- Angers A, Chin J, Cleary LJ, Byrne JH. 5-HT treatment induces redistribution of Aplysia synapsin. Soc Neurosci Abstr. 2000;26:881.
-
- Bähler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–707. - PubMed
-
- Benfenati F, Valtorta F, Chieregatti E, Greengard P. Interaction of free and synaptic vesicle-bound synapsin I with F-actin. Neuron. 1992;8:377–386. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
