Fluorescent Amplified Fragment Length Polymorphism Analysis of Norwegian Bacillus cereus and Bacillus thuringiensis Soil Isolates
- PMID: 11571195
- PMCID: PMC93242
- DOI: 10.1128/AEM.67.10.4863-4873.2001
Fluorescent Amplified Fragment Length Polymorphism Analysis of Norwegian Bacillus cereus and Bacillus thuringiensis Soil Isolates
Abstract
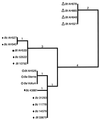
We examined 154 Norwegian B. cereus and B. thuringiensis soil isolates (collected from five different locations), 8 B. cereus and 2 B. thuringiensis reference strains, and 2 Bacillus anthracis strains by using fluorescent amplified fragment length polymorphism (AFLP). We employed a novel fragment identification approach based on a hierarchical agglomerative clustering routine that identifies fragments in an automated fashion. No method is free of error, and we identified the major sources so that experiments can be designed to minimize its effect. Phylogenetic analysis of the fluorescent AFLP results reveals five genetic groups in these group 1 bacilli. The ATCC reference strains were restricted to two of the genetic groups, clearly not representative of the diversity in these bacteria. Both B. anthracis strains analyzed were closely related and affiliated with a B. cereus milk isolate (ATCC 4342) and a B. cereus human pathogenic strain (periodontitis). Across the entire study, pathogenic strains, including B. anthracis, were more closely related to one another than to the environmental isolates. Eight strains representing the five distinct phylogenetic clusters were further analyzed by comparison of their 16S rRNA gene sequences to confirm the phylogenetic status of these groups. This analysis was consistent with the AFLP analysis, although of much lower resolution. The innovation of automated genotype analysis by using a replicated and statistical approach to fragment identification will allow very large sample analyses in the future.
Figures





References
-
- Aarts H J M, van Lith L A J T, Keijer J. High-resolution genotyping of Salmonella strains by AFLP-fingerprinting. Lett Appl Microbiol. 1998;26:131–135. - PubMed
-
- Ash C, Farrow J A, Dorsch M, Stackebrandt E, Collins M D. Comparative analysis of Bacillus anthracis, Bacillus cereus, and related species on the basis of reverse transcriptase sequencing of 16S rRNA. Int J Syst Bacteriol. 1991;41:343–346. - PubMed
-
- Ash C, Farrow J A E, Wallbanks S, Collins M D. Phylogenetic heterogeneity of the genus Bacillus revealed by comparative analysis of small subunit ribosomal RNA sequences. Lett Appl Microbiol. 1991;13:202–206.
-
- Baumann L, Okamoto K, Unterman B M, Lynch M J, Baumann P. Phenotypic characterization of Bacillus thuringiensis and B. cereus. J Invertebr Pathol. 1984;44:329–341.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

