Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans
- PMID: 11172068
- PMCID: PMC29374
- DOI: 10.1073/pnas.98.4.2017
Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans
Abstract
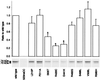
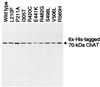
Choline acetyltransferase (ChAT; EC ) catalyzes the reversible synthesis of acetylcholine (ACh) from acetyl CoA and choline at cholinergic synapses. Mutations in genes encoding ChAT affecting motility exist in Caenorhabditis elegans and Drosophila, but no CHAT mutations have been observed in humans to date. Here we report that mutations in CHAT cause a congenital myasthenic syndrome associated with frequently fatal episodes of apnea (CMS-EA). Studies of the neuromuscular junction in this disease show a stimulation-dependent decrease of the amplitude of the miniature endplate potential and no deficiency of the ACh receptor. These findings point to a defect in ACh resynthesis or vesicular filling and to CHAT as one of the candidate genes. Direct sequencing of CHAT reveals 10 recessive mutations in five patients with CMS-EA. One mutation (523insCC) is a frameshifting null mutation. Three mutations (I305T, R420C, and E441K) markedly reduce ChAT expression in COS cells. Kinetic studies of nine bacterially expressed ChAT mutants demonstrate that one mutant (E441K) lacks catalytic activity, and eight mutants (L210P, P211A, I305T, R420C, R482G, S498L, V506L, and R560H) have significantly impaired catalytic efficiencies.
Figures
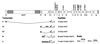

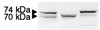
References
-
- Engel A G, Ohno K, Sine S M. In: Myasthenia Gravis and Myasthenic Disorders. Engel AG, editor. New York: Oxford Univ. Press; 1999. pp. 251–297.
-
- Greer M, Schotland M. Pediatrics. 1960;26:101–108. - PubMed
-
- Conomy J P, Levisohn M, Fanaroff A. J Pediatr (Berlin) 1975;87:428–429. - PubMed
-
- Fenichel G M. Arch Neurol (Chicago) 1978;35:97–103. - PubMed
-
- Robertson W C, Chun R W M, Kornguth S E. Arch Neurol (Chicago) 1980;37:117–119. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

