Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties
- PMID: 11319109
- PMCID: PMC92864
- DOI: 10.1128/AEM.67.5.2255-2262.2001
Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties
Abstract
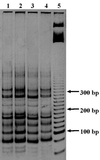
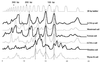
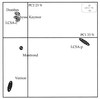
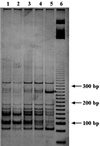
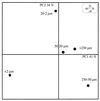
The similarities and differences in the structures of the nifH gene pools of six different soils (Montrond, LCSA-p, Vernon, Dombes, LCSA-c, and Thysse Kaymor) and five soil fractions extracted from LCSA-c were studied. Bacterial DNA was directly extracted from the soils, and a region of the nifH gene was amplified by PCR and analyzed by restriction. Soils were selected on the basis of differences in soil management, plant cover, and major physicochemical properties. Microenvironments differed on the basis of the sizes of the constituent particles and the organic carbon and clay contents. Restriction profiles were subjected to principal-component analysis. We showed that the composition of the diazotrophic communities varied both on a large scale (among soils) and on a microscale (among microenvironments in LCSA-c soil). Soil management seemed to be the major parameter influencing differences in the nifH gene pool structure among soils by controlling inorganic nitrogen content and its variation. However, physicochemical parameters (texture and total C and N contents) were found to correlate with differences among nifH gene pools on a microscale. We hypothesize that the observed nifH genetic structures resulted from the adaptation to fluctuating conditions (cultivated soil, forest soil, coarse fractions) or constant conditions (permanent pasture soil, fine fractions). We attempted to identify a specific band within the profile of the clay fraction by cloning and sequencing it and comparing it with the gene databases. Unexpectedly, the nifH sequences of the dominant bacteria were most similar to sequences of unidentified marine eubacteria.
Figures

References
-
- Achouak W, Normand P, Heulin T. Comparative phylogeny of rrs and nifH genes in the Bacillaceae. Int J Syst Bacteriol. 1999;49:961–967. - PubMed
-
- Alexander M, editor. Microbial ecology. New York, N.Y: John Wiley & Sons, Inc.; 1971.
-
- Atlas R M, Bartha R. Microbial ecology. Fundamentals and applications. Reading, Mass: Addison-Wesley Publishing Co.; 1981.
-
- Balesdent J, Mariotti A, Boisgontier D. Effect of tillage on soil organic carbon mineralization estimated from 13C abundance in maize fields. J Soil Sci. 1990;41:587–596.
-
- Bardgett R D, Mawdsley J L, Edwards S, Hobbs P J, Rodwell J S, Davies W J. Plant species and nitrogen effects on soil biological properties of template upland grasslands. Funct Ecol. 1999;13:650–660.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

