Two alpha(1-3) glucan synthases with different functions in Aspergillus fumigatus
- PMID: 15746357
- PMCID: PMC1065186
- DOI: 10.1128/AEM.71.3.1531-1538.2005
Two alpha(1-3) glucan synthases with different functions in Aspergillus fumigatus
Abstract
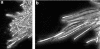
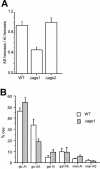
Alpha(1-3) glucan is a main component of the Aspergillus fumigatus cell wall. In spite of its importance, synthesis of this amorphous polymer has not been investigated to date. Two genes in A. fumigatus, AGS1 and AGS2, are highly homologous to the AGS genes of Schizosaccharomyces pombe, which encode putative alpha(1-3) glucan synthases. The predicted Ags proteins of A. fumigatus have an estimated molecular mass of 270 kDa. AGS1 and AGS2 were disrupted in A. fumigatus. Both Deltaags mutants have similar altered hyphal morphologies and reduced conidiation levels. Only Deltaags1 presented a reduction in the alpha(1-3) glucan content of the cell wall. These results showed that Ags1p and Ags2p were functionally different. The cellular localization of the two proteins was in agreement with their different functions: Ags1p was localized at the periphery of the cell in connection with the cell wall, whereas Ags2p was intracellularly located. An original experimental model of invasive aspergillosis based on mixed infection and quantitative PCR was developed to analyze the virulence of A. fumigatus mutant and wild-type strains. Using this model, it was shown that the cell wall and morphogenesis defects of Deltaags1 and Deltaags2 were not associated with a reduction in virulence in either mutant. This result showed that a 50% reduction in the content of the cell wall alpha(1-3) glucan does not play a significant role in A. fumigatus pathogenicity.
Figures


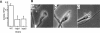

References
-
- Beauvais, A., and J. P. Latgé. 2001. Membrane and cell wall targets in Aspergillus fumigatus. Drug Resist. Updates. 4:1-12. - PubMed
-
- Brian, P. W., A. W. Dawkins, J. F. Grove, H. G. Hemming, D. Lowe, and G. L. Norris. 1961. Phytotoxic compounds produced by Fusarium equiseti. J. Exp. Bot. 12:1-7.
-
- Claros, M. G., and G. van Heijne. 1994. TopRed II: an improved software for membrane protein structure predictions. Comput. Appl. Biol. Sci. 10:685-686. - PubMed
-
- Dijkgraaf, G. J. P., M. Abe, Y. Ohya, and H. Bussey. 2002. Mutations in Fks1p affect the cell wall content of β-1,3- and β-1,6-glucan in Saccharomyces cerevisiae. Yeast 19:671-690. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

