Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175
- PMID: 11309486
- PMCID: PMC33191
- DOI: 10.1073/pnas.081075398
Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175
Abstract
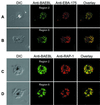
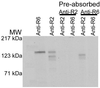
A member of a Plasmodium receptor family for erythrocyte invasion was identified on chromosome 13 from the Plasmodium falciparum genome sequence of the Sanger Centre (Cambridge, U.K.). The protein (named BAEBL) has homology to EBA-175, a P. falciparum receptor that binds specifically to sialic acid and the peptide backbone of glycophorin A on erythrocytes. Both EBA-175 and BAEBL localize to the micronemes, organelles at the invasive ends of the parasites that contain other members of the family. Like EBA-175, the erythrocyte receptor for BAEBL is destroyed by neuraminidase and trypsin, indicating that the erythrocyte receptor is a sialoglycoprotein. Its specificity, however, differs from that of EBA-175 in that BAEBL can bind to erythrocytes that lack glycophorin A, the receptor for EBA-175. It has reduced binding to erythrocytes with the Gerbich mutation found in another erythrocyte, sialoglycoprotein (glycophorin C/D). The interest in BAEBL's reduced binding to Gerbich erythrocytes derives from the high frequency of the Gerbich phenotype in some regions of Papua New Guinea where P. falciparum is hyperendemic.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

