Staphylococcus intermedius produces a functional agr autoinducing peptide containing a cyclic lactone
- PMID: 15838041
- PMCID: PMC1082806
- DOI: 10.1128/JB.187.9.3139-3150.2005
Staphylococcus intermedius produces a functional agr autoinducing peptide containing a cyclic lactone
Abstract
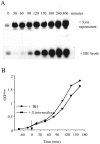
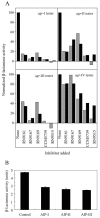
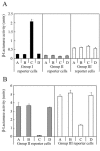
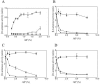
The agr system is a global regulator of accessory functions in staphylococci, including genes encoding exoproteins involved in virulence. The agr locus contains a two-component signal transduction module that is activated by an autoinducing peptide (AIP) encoded within the agr locus and is conserved throughout the genus. The AIP has an unusual partially cyclic structure that is essential for function and that, in all but one case, involves an internal thiolactone bond between a conserved cysteine and the C-terminal carboxyl group. The exceptional case is a strain of Staphylococcus intermedius that has a serine in place of the conserved cysteine. We demonstrate here that the S. intermedius AIP is processed by the S. intermedius AgrB protein to generate a cyclic lactone, that it is an autoinducer as well as a cross-inhibitor, and that all of five other S. intermedius strains examined also produce serine-containing AIPs.
Figures




References
-
- Fitton, J. E., A. Dell, and W. V. Shaw. 1980. The amino acid sequence of the delta haemolysin of Staphylococcus aureus. FEBS Lett. 115:209-212. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

