Functionally important glycosyltransferase gain and loss during catarrhine primate emergence
- PMID: 17194757
- PMCID: PMC1766424
- DOI: 10.1073/pnas.0610012104
Functionally important glycosyltransferase gain and loss during catarrhine primate emergence
Abstract
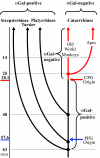
A glycosyltransferase, alpha1,3galactosyltransferase, catalyzes the terminal step in biosynthesis of Galalpha1,3Galbeta1-4GlcNAc-R (alphaGal), an oligosaccharide cell surface epitope. This epitope or antigenically similar epitopes are widely distributed among the different forms of life. Although abundant in most mammals, alphaGal is not normally found in catarrhine primates (Old World monkeys and apes, including humans), all of which produce anti-alphaGal antibodies from infancy onward. Natural selection favoring enhanced resistance to alphaGal-positive pathogens has been the primary reason offered to account for the loss of alphaGal in catarrhines. Here, we question the primacy of this immune defense hypothesis with results that elucidate the evolutionary history of GGTA1 gene and pseudogene loci. One such locus, GGTA1P, a processed (intronless) pseudogene (PPG), is present in platyrrhines, i.e., New World monkeys, and catarrhines but not in prosimians. PPG arose in an early ancestor of anthropoids (catarrhines and platyrrhines), and GGTA1 itself became an unprocessed pseudogene in the late catarrhine stem lineage. Strong purifying selection, denoted by low nonsynonymous substitutions per nonsynonymous site/synonymous substitutions per synonymous site values, preserved GGTA1 in noncatarrhine mammals, indicating that the functional gene product is subjected to considerable physiological constraint. Thus, we propose that a pattern of alternative and/or more beneficial glycosyltransferase activity had to first evolve in the stem catarrhines before GGTA1 inactivation could occur. Enhanced defense against alphaGal-positive pathogens could then have accelerated the replacement of alphaGal-positive catarrhines by alphaGal-negative catarrhines. However, we emphasize that positively selected regulatory changes in sugar chain metabolism might well have contributed in a major way to catarrhine origins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Martin RD. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton: Princeton Univ Press; 1990.
-
- Harvey PH, Martin RD, Clutton-Brock TH. In: Primate Societies. Smuts BB, Cheyney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Chicago: Univ of Chicago Press; 1987. pp. 181–196.
-
- Dominy NJ, Lucas PW. Nature. 2001;410:363–366. - PubMed
-
- Goodman M, Porter CA, Czelusniak J, Page SL, Schneider H, Shoshani J, Gunnell G, Groves CP. Mol Phylogenet Evol. 1998;9:585–598. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

