Monitoring impact of a pesticide treatment on bacterial soil communities by metabolic and genetic fingerprinting in addition to conventional testing procedures
- PMID: 9687435
- PMCID: PMC106777
- DOI: 10.1128/AEM.64.8.2814-2821.1998
Monitoring impact of a pesticide treatment on bacterial soil communities by metabolic and genetic fingerprinting in addition to conventional testing procedures
Abstract
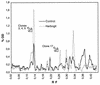
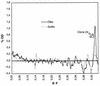
Herbogil (dinoterb), a reference herbicide, the mineral oil Oleo (paraffin oil used as an additive to herbicides), and Goltix (metamitron) were taken as model compounds for the study of impacts on microbial soil communities. After the treatment of soil samples, effects on metabolic sum parameters were determined by monitoring substrate-induced respiration (SIR) and dehydrogenase activity, as well as carbon and nitrogen mineralization. These conventional ecotoxicological testing procedures are used in pesticide registration. Inhibition of biomass-related activities and stimulation of nitrogen mineralization were the most significant effects caused by the application of Herbogil. Even though Goltix and Oleo were used at a higher dosage (10 times higher), the application of Goltix resulted in smaller effects and the additive Oleo was the least-active compound, with minor stimulation of test parameters at later observation times. The results served as a background for investigation of the power of "fingerprinting" methods in microbial ecology. Changes in catabolic activities induced by treatments were analyzed by using the 95 carbon sources provided by the BIOLOG system. Variations in the complex metabolic fingerprints demonstrated inhibition of many catabolic pathways after the application of Herbogil. Again, the effects of the other compounds were expressed at much lower levels and comprised stimulations as well as inhibitions. Testing for significance by a multivariate t test indicated that the sensitivity of this method was similar to the sensitivities of the conventional testing procedures. The variation of sensitive carbon sources, as determined by factor weights at different observation times, indicated the dynamics of the community shift induced by the Herbogil treatment in more detail. DNA extractions from soil resulted in a collection of molecules representing the genetic composition of total bacterial communities. Distinct and highly reproducible community patterns, or genetic fingerprints, resulting from application of the different herbicides were obtained by the sequence-specific separation of partial 16S rDNA amplification products in temperature gradient gel electrophoresis. Significant pattern variations were quantified. For detailed analysis, application-responsive bands from the Herbogil and Oleo treatments were sequenced and their tentative phylogenetic positions were identified. Data interpretation and the potentials and biases of the additional observation windows on microbial communities are discussed.
Figures




References
-
- Anderson J P E, Domsch K H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem. 1978;10:215–221.
-
- Anderson J P E, Castle D, Ehle H, Eichler D, Laermann H-T, Maas G, Malkomes H-P. Guideline for the official testing of plant protection products. 2nd ed., part VI, 1-1. Effects on the activity of the soil microflora. Biologische Bundesanstalt für Land- und Forstwirtschaft. Germany: Braunschweig; 1990. . (In German.)
-
- Atlas R M. Diversity of microbial communities. Adv Microb Ecol. 1984;7:1–47.
-
- Atlas R M. Use of microbial diversity measurements to assess environmental stress. In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Washington, D.C: American Society for Microbiology; 1984. pp. 540–545.
-
- Belkin S, Stieber M, Thiem A, Frimmel F H, Abeliovitch A, Werner P, Ulitzur S. Toxicity and genotoxicity enhancement during polycyclic aromatic hydrocarbons’ biodegradation. Environ Toxicol Water Qual. 1994;9:303–309.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

