Adjuvant nab-Paclitaxel + Gemcitabine in Resected Pancreatic Ductal Adenocarcinoma: Results From a Randomized, Open-Label, Phase III Trial
- PMID: 36521097
- PMCID: PMC10082313
- DOI: 10.1200/JCO.22.01134
Adjuvant nab-Paclitaxel + Gemcitabine in Resected Pancreatic Ductal Adenocarcinoma: Results From a Randomized, Open-Label, Phase III Trial
Abstract
Purpose: This randomized, open-label trial compared the efficacy and safety of adjuvant nab-paclitaxel + gemcitabine with those of gemcitabine for resected pancreatic ductal adenocarcinoma (ClinicalTrials.gov identifier: NCT01964430).
Methods: We assigned 866 treatment-naive patients with pancreatic ductal adenocarcinoma to nab-paclitaxel (125 mg/m2) + gemcitabine (1,000 mg/m2) or gemcitabine alone to one 30-40 infusion on days 1, 8, and 15 of six 28-day cycles. The primary end point was independently assessed disease-free survival (DFS). Additional end points included investigator-assessed DFS, overall survival (OS), and safety.
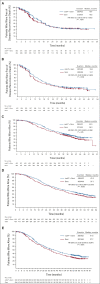
Results: Two hundred eighty-seven of 432 patients and 310 of 434 patients completed nab-paclitaxel + gemcitabine and gemcitabine treatment, respectively. At primary data cutoff (December 31, 2018; median follow-up, 38.5 [interquartile range [IQR], 33.8-43 months), the median independently assessed DFS was 19.4 (nab-paclitaxel + gemcitabine) versus 18.8 months (gemcitabine; hazard ratio [HR], 0.88; 95% CI, 0.729 to 1.063; P = .18). The median investigator-assessed DFS was 16.6 (IQR, 8.4-47.0) and 13.7 (IQR, 8.3-44.1) months, respectively (HR, 0.82; 95% CI, 0.694 to 0.965; P = .02). The median OS (427 events; 68% mature) was 40.5 (IQR, 20.7 to not reached) and 36.2 (IQR, 17.7-53.3) months, respectively (HR, 0.82; 95% CI, 0.680 to 0.996; P = .045). At a 16-month follow-up (cutoff, April 3, 2020; median follow-up, 51.4 months [IQR, 47.0-57.0]), the median OS (511 events; 81% mature) was 41.8 (nab-paclitaxel + gemcitabine) versus 37.7 months (gemcitabine; HR, 0.82; 95% CI, 0.687 to 0.973; P = .0232). At the 5-year follow-up (cutoff, April 9, 2021; median follow-up, 63.2 months [IQR, 60.1-68.7]), the median OS (555 events; 88% mature) was 41.8 versus 37.7 months, respectively (HR, 0.80; 95% CI, 0.678 to 0.947; P = .0091). Eighty-six percent (nab-paclitaxel + gemcitabine) and 68% (gemcitabine) of patients experienced grade ≥ 3 treatment-emergent adverse events. Two patients per study arm died of treatment-emergent adverse events.
Conclusion: The primary end point (independently assessed DFS) was not met despite favorable OS seen with nab-paclitaxel + gemcitabine.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

Comment in
-
Adjuvant Gemcitabine and Nab-Paclitaxel Misses the Target in Pancreas Adenocarcinoma: Or Did an Effective Therapy Fall to the Definition of Recurrence?J Clin Oncol. 2023 Apr 10;41(11):1972-1975. doi: 10.1200/JCO.23.00039. Epub 2023 Feb 28. J Clin Oncol. 2023. PMID: 36854094 No abstract available.
References
-
- World Health Organization : Latest world cancer statistics—GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. https://www.iarc.fr/news-events/latest-world-cancer-statistics-globocan-...
-
- Ducreux M, Cuhna AS, Caramella C, et al. : Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v56-v68, 2015. (suppl 5) - PubMed
-
- National Cancer Institute—Surveillance, Epidemiology, and End Results Program : Cancer Stat Facts: Pancreatic Cancer. 2018. https://seer.cancer.gov/statfacts/html/pancreas.html
-
- American Cancer Society : Cancer Facts and Figures. 2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-...
-
- Groot VP, van Santvoort HC, Rombouts SJ, et al. : Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; re-resection, chemoradiotherapy and SBRT. HPB (Oxford) 19:83-92, 2017 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

