Aspirin as secondary prevention in colorectal cancer liver metastasis (ASAC trial): study protocol for a multicentre randomized placebo-controlled trial
- PMID: 34544470
- PMCID: PMC8451095
- DOI: 10.1186/s13063-021-05587-w
Aspirin as secondary prevention in colorectal cancer liver metastasis (ASAC trial): study protocol for a multicentre randomized placebo-controlled trial
Abstract
Background: Colorectal cancer is one the most common cancers in the western world with increasing incidence. Approximately 50% of the patients develop liver metastases. Resection of liver metastases is the treatment of choice although almost half of the resected patients get recurrence in the liver.
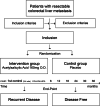
Methods: The ASAC trial is a Scandinavian, multicentre, double-blinded, randomized, placebo-controlled study to determine whether adjuvant treatment with low-dose aspirin (acetylsalicylic acid (ASA)) can improve disease-free survival in patients treated for colorectal cancer liver metastases (CRCLM). Up to 800 patients operated for CRCLM will be randomized to Arm#1 ASA 160 mg once daily or Arm#2 Placebo, for a period of 3 years or until disease recurrence. The patients will be recruited at all major hepatobiliary surgical units in Norway, Sweden and Denmark and have follow-up according to standard of care and the National Guidelines.
Discussion: The ASAC trial will be the first clinical interventional trial to assess the potential beneficial role of ASA in recurrence of CRCLM and survival. ASA is an inexpensive, well-tolerated and easily accessible drug that will be highly potential as adjuvant drug in secondary prevention of CRCLM if the study shows a beneficial effect. We will also determine the effect of ASA as adjuvant treatment on Health-Related Quality of Life and the cost-effectiveness.
Trial registration: ClinicalTrials.gov NCT03326791 . Registered on 31 October 2017.
Keywords: Acetylsalicylic acid; Aspirin; Colorectal cancer; Liver metastases; Secondary prevention.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Scherman P, Syk I, Holmberg E, Naredi P, Rizell M. Impact of patient, primary tumor and metastatic pattern including tumor location on survival in patients undergoing ablation or resection for colorectal liver metastases: a population-based national cohort study. Eur J Surg Oncol. 2021;47(2):375–83. 10.1016/j.ejso.2020.07.030. - PubMed
-
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, Allen JI, Beach M, Beck GJ, Bond JH, Byers T, Greenberg ER, Mandel JS, Marcon N, Mott LA, Pearson L, Saibil F, van Stolk RU. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. - DOI - PubMed