Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study
- PMID: 19070889
- PMCID: PMC2646126
- DOI: 10.1016/S0140-6736(08)61766-3
Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study
Erratum in
- Lancet. 2009 May 23;373(9677):1764
Abstract
Background: Hysterectomy and bilateral salpingo-oophorectomy (BSO) is the standard surgery for stage I endometrial cancer. Systematic pelvic lymphadenectomy has been used to establish whether there is extra-uterine disease and as a therapeutic procedure; however, randomised trials need to be done to assess therapeutic efficacy. The ASTEC surgical trial investigated whether pelvic lymphadenectomy could improve survival of women with endometrial cancer.
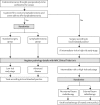
Methods: From 85 centres in four countries, 1408 women with histologically proven endometrial carcinoma thought preoperatively to be confined to the corpus were randomly allocated by a minimisation method to standard surgery (hysterectomy and BSO, peritoneal washings, and palpation of para-aortic nodes; n=704) or standard surgery plus lymphadenectomy (n=704). The primary outcome measure was overall survival. To control for postsurgical treatment, women with early-stage disease at intermediate or high risk of recurrence were randomised (independent of lymph-node status) into the ASTEC radiotherapy trial. Analysis was by intention to treat. This study is registered, number ISRCTN 16571884.
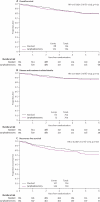
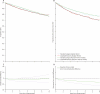
Findings: After a median follow-up of 37 months (IQR 24-58), 191 women (88 standard surgery group, 103 lymphadenectomy group) had died, with a hazard ratio (HR) of 1.16 (95% CI 0.87-1.54; p=0.31) in favour of standard surgery and an absolute difference in 5-year overall survival of 1% (95% CI -4 to 6). 251 women died or had recurrent disease (107 standard surgery group, 144 lymphadenectomy group), with an HR of 1.35 (1.06-1.73; p=0.017) in favour of standard surgery and an absolute difference in 5-year recurrence-free survival of 6% (1-12). With adjustment for baseline characteristics and pathology details, the HR for overall survival was 1.04 (0.74-1.45; p=0.83) and for recurrence-free survival was 1.25 (0.93-1.66; p=0.14).
Interpretation: Our results show no evidence of benefit in terms of overall or recurrence-free survival for pelvic lymphadenectomy in women with early endometrial cancer. Pelvic lymphadenectomy cannot be recommended as routine procedure for therapeutic purposes outside of clinical trials.
Figures


Comment in
-
Treatment of early endometrial carcinoma: is less more?Lancet. 2009 Jan 10;373(9658):97-9. doi: 10.1016/S0140-6736(08)61768-7. Epub 2008 Dec 16. Lancet. 2009. PMID: 19070890 No abstract available.
-
Lymphadenectomy in endometrial cancer.Lancet. 2009 Apr 4;373(9670):1169-70; author reply 1170-1. doi: 10.1016/S0140-6736(09)60676-0. Lancet. 2009. PMID: 19345819 No abstract available.
-
Lymphadenectomy in endometrial cancer.Lancet. 2009 Apr 4;373(9670):1169; author reply 1170-1. doi: 10.1016/S0140-6736(09)60675-9. Lancet. 2009. PMID: 19345820 No abstract available.
-
Lymphadenectomy in endometrial cancer.Lancet. 2009 Apr 4;373(9670):1169; author reply 1170-1. doi: 10.1016/S0140-6736(09)60674-7. Lancet. 2009. PMID: 19345821 No abstract available.
-
Lymphadenectomy in endometrial cancer.Lancet. 2009 Apr 4;373(9670):1170; author reply 1170-1. doi: 10.1016/S0140-6736(09)60677-2. Lancet. 2009. PMID: 19345823 No abstract available.
-
Evaluation of the efficacy of systematic pelvic lymphadenectomy in endometrial cancer.Future Oncol. 2009 May;5(4):459-63. doi: 10.2217/fon.09.19. Future Oncol. 2009. PMID: 19450175 No abstract available.
References
-
- Cancer Research UK CancerStats: corpus uteri cancer. http://info.cancerresearchuk.org/cancerstats/ (accessed Aug 5, 2008).
-
- Boyle P, Leon ME, Maisonneuve P, Autier P. Cancer control in women. Update 2003. Int J Gynaecol Obstet. 2003;83(suppl 1):179–202. - PubMed
-
- Cancer facts and figures 2008. American Cancer Society; Atlanta: 2006. Available at http://seer.cancer.gov/csr/1975_2005/results_single/sect_01... (accessed Aug 5, 2008).
-
- Sant M, Aareleid T, Berrino F. EUROCARE-3: survival of cancer patients diagnosed 1990–94—results and commentary. Ann Oncol. 2003;14(suppl 5):v61–118. - PubMed
-
- Boronow RC, Morrow CP, Creasman WT. Surgical staging in endometrial cancer: clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984;63:825–832. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

